stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
the most beautiful residue
see 4-fluoroamphetamine residue in the pic.
Picture removed - Too large - Resize to 800*600 and try again
[Edited on 11-1-2007 by vulture]
[Edited on 11-1-2007 by vulture]
|
|
|
Douchermann
Hazard to Others
  
Posts: 117
Registered: 11-10-2005
Location: Illinois, USA
Member Is Offline
Mood: No Mood
|
|
wow thats pretty awesome.
It\'s better to be pissed off than to be pissed on.
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
No way....it looks like a christmas decoration.
That is very awesome. 
[Edited on 4/30/2006 by lacrima97]
|
|
|
chochu3
Hazard to Others
  
Posts: 185
Registered: 21-10-2003
Location: South Side Tejas [Cloverland]
Member Is Offline
Mood: Inside looking out
|
|
neat
\"Abiding in the midst of ignorance, thinking themselves wise and learned, fools go aimlessly hither and thither, like blind led by the blind.\" -
Katha Upanishad
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
Almost too pretty to be real. Bottom of an erlenmeyer?
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
no, RBF bottom. i take this as a sign...substances of beauty will do the mind good 
|
|
|
Vitus_Verdegast
Hazard to Others
  
Posts: 297
Registered: 5-12-2004
Location: Ottoman Empire
Member Is Offline
Mood: tea time
|
|
Very beautiful and impressive!
Reminds me strongly of 1920's art deco style
(eg. the inside decoration of artisanally bound volumes of the chemical journals of those days)
I ought to show this to my artist friend, he'll be impressed.
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
Idk, I think I may have found some crystal residue which is even more beautiful, or at least rivals it.
http://img.photobucket.com/albums/v58/nitric63/Crystals003.j...
http://img.photobucket.com/albums/v58/nitric63/Crystals8.jpg
http://img.photobucket.com/albums/v58/nitric63/Crystals9.jpg
The second two images are obviously just manipulations of the first using paint. The third one is now my computer's background.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Oh yes, acicular "bunches of wheat" formations. I've had some of that from CuCl2 for instance, and PbAc2. Nice stuff 8)
Tim
|
|
|
chems
Harmless

Posts: 1
Registered: 28-5-2006
Member Is Offline
Mood: No Mood
|
|
4-fluoroamphetamine as a analog of amphetamine, neurotoxic reagent, controled to be used. Those toxic things are beautiful as well?
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
In how far 4-FA is neurotoxic is not clear but it has for sure a more toxic feel then amphetamine and methamphetamine. And less bang.
/ORG
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
| Quote: | Originally posted by Organikum
In how far 4-FA is neurotoxic is not clear but it has for sure a more toxic feel then amphetamine and methamphetamine. And less bang.
/ORG |
how would you describe feeling the effects of toxicity? for most people, 4-fa actually feels a lot less toxic than methamphetamine. it sure is not
quite as potent as the latter, but the quality of its effects is far off from plain (meth)amphetamine. somehow, it has a lingering mood-brightening
effect for several days after ingestion.
|
|
|
bhassi
Harmless

Posts: 12
Registered: 19-4-2006
Member Is Offline
Mood: No Mood
|
|
who says that beauty only lies in the eyes of sweet girls.......
|
|
|
Vitus_Verdegast
Hazard to Others
  
Posts: 297
Registered: 5-12-2004
Location: Ottoman Empire
Member Is Offline
Mood: tea time
|
|
4-FA: not neurotoxic in reasonable doses in animals (rats?)according to the literature (IIRC). The other 4-halogenated amphetamines are very
neurotoxic (cause long-term irreversible 5HT depletion and results in degeneration of serotonergic axons).
But 4-FA has serotonergic effects, which should make it feel differently than unsubstituted amphetamine. An acquintance of mine once had a severe
depression which lasted for a couple of days after using only moderate amounts of 4-FA sulfate via insufflation. He seemed to suspect a serotonine
related issue as the cause of this.
From http://tinyurl.com/gvxgf :
| Quote: |
Both compounds increased locomotor activity 10 min after injection, but FAM had sedative effects after 1 h, while AM continued to be stimulatory.
|
2-fluoroamphetamine is rumoured to be more similar to amphetamine than 4-FA.
Beautiful crystals:
Does anyone have more pictures to add? 
[Edited on 5-6-2006 by Vitus_Verdegast]
Sic transit gloria mundi
|
|
|
Margaret_Thatcher
Harmless

Posts: 1
Registered: 5-6-2006
Member Is Offline
Mood: No Mood
|
|
Yes, Dennis sampled this several times and reported a strangely mellow and long-lasting high. In fact, Dennis reported no unpleasant after effects -
far superior to MA but not quite as satisfying as a large scotch.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Vitus_Verdegast
4-FA: not neurotoxic in reasonable doses in animals (rats?)according to the literature (IIRC). The other 4-halogenated amphetamines are very
neurotoxic (cause long-term irreversible 5HT depletion and results in degeneration of serotonergic axons). |
What about the 4-NO2 derivate?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
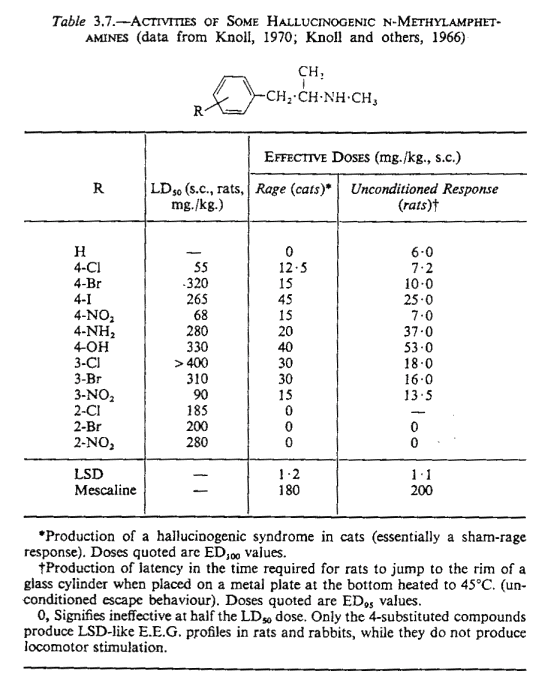
I don't know about p-nitroamphetamine, but N-methyl-p-nitroamphetamine was studied in certain animal models. It showed very high potency, but had also
a considerably low LD50. Though the animal models were claimed to be showing hallucinogenic potency mind that this was performed at the end of the
60's when there were no generalization studies or other models that could show psychedelic or other specific activity with at least some certainty.
So, all these tests showed is that N-methyl-p-nitroamphetamine is a very potent psychoactive agent with an unfavorable therapeutic index but nothing
more specific than this. As far as to neurotoxicity goes I can only guess. Para nitro is not the same as para chloro, bromo or iodo groups that make
neurotoxic amphetamines. If it was only for just any para substitution than those few that use p-methyl(meth)amphetamine might also worry but it seams
they don’t.
Anyhow, it is not easy to prepare p-nitroamphetamine without risking going trough amphetamine which is illegal essentially all over the world. There
are only limited reaction pathways that would be fully legal all the way to the product. One would be to somehow prepare p-nitrophenyacetone (by
acylating p-nitrotoluene?) and reductively aminate it with NaCNBH3 or other appropriate reagents that would leave the nitro group intact.
References regarding N-methyl-p-nitroamphetamine:
Knoll, J. (1970), in Amphetamines and Related Compounds (ed. Costa and Garattini), pp. 761-780. New York: Raven Press.
Knoll, J., and Vizi, E. S. (1970), Psychopharmacologia, 4,278.
Knoll, J., Vizi, E. S., and Ecseri, Z. (1966), Arch. int. Pharmacodyn. Ther., 159, 448.
Knoll, J., Vizi, E. S., and Knoll, B. (1970), Acta Physiol. Acad. Sci. Hung., 37,151.
Knoll, J., Vizi, E. S., and Knoll, B. (1971), Proc. Eur. Soc. Study Drug Toxic., 12, 50.
Check the attached table from Hallucinogenic agents by Brimblecombe and Pinder (Bristol: Wright-Scientechnica, 1975).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Vitus_Verdegast
Hazard to Others
  
Posts: 297
Registered: 5-12-2004
Location: Ottoman Empire
Member Is Offline
Mood: tea time
|
|
Errandum:
| Quote: |
Both compounds increased locomotor activity 10 min after injection, but FAM had sedative effects after 1 h, while AM continued to be stimulatory.
|
In my hurry I didn't see that the paper mentioned discusses the amphetamine analog with the fluorine on the aliphatic alpha-position (CH2F)
instead of the para position on the ring.
Nevertheless IMO the above description fits pretty well for 4-FA.
Concerning 2-FA: I've heard in the days of the Hive a sampler report through private communication that the effects resembled methamphetamine, were
long lasting, but with a distinct hallucinogenic character ("trippy").
Unfortunately my usual chemical suppliers charges 80 euro/25 ml for 2-F-benzaldehyde (&!§$$!%£..thieves.. .) .)
Fluorobenzene is dirt cheap, it would be interesting to find a route from there. Why not chloromethylation, followed by hexamine treatment to the
aldehyde? Mixtures of 2-F- and 4-F-benzaldehyde should be separated by their difference in melting points (2-F-BA = -44°C ; 4-F-BA = -10°C)..
[Edited on 6-6-2006 by Vitus_Verdegast]
Sic transit gloria mundi
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Attachment: monosubstituted_MA.gif (37.97kb). |
Strange results, I will have to think about that...
| Quote: | | Why not chloromethylation, followed by hexamine treatment to the aldehyde? Mixtures of 2-F- and 4-F-benzaldehyde should be separated by their
difference in melting points (2-F-BA = -44°C ; 4-F-BA = -10°C).. |
Or react the grignard reagent with acetonitrile and reduce the intermediate nitrile.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
More references...
Apparently p-nitro-N-methylamphetamine is a monoamine releaser, but in contrast to plain amphetamine it releases serotonin as well. See:
Leonard, B. E. Effect of four amphetamines on brain biogenic amines and their metabolites. Biochemical Pharmacology, 21(9),
1289-1297 (1972).
Leonard, B. E.; Shallice, Susan A. Effect of p-nitromethylamphetamine on biogenic amines and their amino acid precursors in rat
brain. British Journal of Pharmacology, 43(4), 732-738 (1971).
Pletscher, A.; Da Prada, M.; Bartholini, G.; Burkard, W. P. Differential influence on the metabolism of endogenous aromatic monoamines of
substituted aralkylamines. Helvetica Physiologica et Pharmacologica Acta, 23(4) , C102-C104 (1965). (written in
German)
This might explain the putative »hallucinogenic« activity described by Knoll in his unreliable animal models. It is thus not really a hallucinogen
or one could classify other similar monoamine releasers, like MDMA for example, as hallucinogens as well, but obviously they are not (at least not
psychedelic in nature).
Surprisingly, I found no references on the pharmacology of the p-nitroamphetamine. So, Turd, I’m afraid your original question remains unanswered.
However, given the more or less known SAR theory of these monoamine releasers (like p-X-(M)A where X=halogen, MD(M)A, MMA, indan-5-yl-isopropylamine
etc.) the N-methylation only changes the selectivity between dopamine, noradrenalin and serotonin releasing while both type of compounds retain the
monoamine releasing property.
I have also not found any legal route to either p-nitroamphetamine or p-nitro-N-methylamphetamine in the published literature. All articles use the
nitration of (methyl)amphetamine with nitric acid. This one is the most exemplary:
Patrick, T. M., Jr.; McBee  , E. T.; Hass, H. B. Synthesis of
arylpropylamines. III. From nuclear nitration. Journal of the American Chemical Society, 68, 1153-1155 (1946). , E. T.; Hass, H. B. Synthesis of
arylpropylamines. III. From nuclear nitration. Journal of the American Chemical Society, 68, 1153-1155 (1946).
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | | Originally posted by Nicodem Anyhow, it is not easy to prepare p-nitroamphetamine without risking going trough amphetamine which is illegal
essentially all over the world. There are only limited reaction pathways that would be fully legal all the way to the product. One would be to somehow
prepare p-nitrophenyacetone (by acylating p-nitrotoluene?) and reductively aminate it with NaCNBH3 or other appropriate reagents that would leave the
nitro group intact. |
Plain NaBH4 leaves the aromatic nitro intact, IIRC...
Anyhow, I haven't got the acsess to beilstein any longer  but one can easily
make p-nitrobenzyl cyanide and hence also p-nitrophenylacetic acid... but one can easily
make p-nitrobenzyl cyanide and hence also p-nitrophenylacetic acid...
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv1p0396
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv1p0406
Although IMO a better use of the above cyanide would be to react it with ethylacetate under basic condition, hydrolyze and decarboxylate to get the
P2P:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv2p0487
http://www.orgsyn.org/orgsyn/prep.asp?prep=CV2P0389
Note that the methylene hydrogens of the p-nitrobenzyl cyanide are exceptionally acidic, much more so than of plain benzyl cyanide as in the above
prep, probably a even weaker base can be used but I'd rather also try PTC, like here it might work:
http://www.orgsyn.org/orgsyn/prep.asp?prep=cv6p0897
[Edited on 8-1-2007 by Sandmeyer]
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Not to get off topic...Well rather back on topic.
Humble Na2SO4. This stuff leaves some beautiful, lacy white patterns of the anhydrous form when a small puddle of solution doesn't get wiped up or
when an empty beaker is left overnight without washing it. These are crystal shots since I have no pics of the residue. They never form the same way
twice, it seems.

Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|
UnintentionalChaos
International Hazard
    
Posts: 1454
Registered: 9-12-2006
Location: Mars
Member Is Offline
Mood: Nucleophilic
|
|
Sorry about the double post. Still don't have a site to post pics to so I can use the BB code. 

Department of Redundancy Department - Now with paperwork!
'In organic synthesis, we call decomposition products "crap", however this is not a IUPAC approved nomenclature.' -Nicodem
|
|
|