Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Synthesis of para aromatic hydroxyls.
Chemicals like p-hydroxy-benzaldehyde, p-cresol, or 2-naphthol are often needed as building blocks in organic chemistry.
Would the following route work?
By first sulfonating the arene with H2SO4, an para aromatic sulfonic acid is obtained.
Removing the sulfonate group in molten NaOH gives a para aromatic alkoxide.
Acid workup gives the desired para hydroxy arene.
If it does work, it would be an OTC route to para aromatic hydroxyls, assuming that the initial arene is already available.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Welcome to the forum!
p-Cresol, 1- and 2- naphthols are readily prepared by the process of alkali fusion with the sodium salt of the sulphonic acid but I don't think you
can prepare p-(4-)-hydroxybenzaldehyde by this process (it is usually prepared by the thermal rearrangement of salicylaldehyde). I don't think the
aldehyde group will survive such brutal treatment; I think that it will either oxidize or undergro a Cannizzaro like reaction.
This reaction is an industrially important one and has been very well studied. I have numerous classic textbook on the preparation of dye
intermediates and reading them makes it clear that the reaction is complex with many unexpected results. Furthermore it is not a very amateur-friendly
technique. I would suggest you read up about some of the standard laboratory procedures (try Vogel for starters) and consider the equipment you are
going to use. For instance, how are you going to protect the delicate thermometer bulb from the extemely corrosive fused alkali? Do you have a steel
clad thermocouple? etc.
There are several tread on this forum by guys that have used this procedure to prepare phenol or resorcinol. I suggest you read them before attempting
it yourself
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Why would I need a thermometer in the first place, just an IR one should be good enough from a distance?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Maybe a protecting acetal group would stop the Cannizzaro? And what would cause it to oxidize?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
p hydroxybenzaldehyde was my main goal in this synthesis, so I want to find a route to that.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Most methods of aldehyde formation (formylation) from phenol yield the p- isomer. The Rimmer Tiemann reaction is an exception but apparently you can
convert the o- isomer into the p-isomer. There are vast numbers of references to its preparation, including many patents.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
P-hydroxy benzaldehyde may be obtained by the cleavage of anethole to p-methoxybenzaldehyde and demethylation — these reactions are somewhat
well-developed among amateur chemists
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Can you put a link to the forum with the procedures? I couldn't find any
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by Boffis  | | Most methods of aldehyde formation (formylation) from phenol yield the p- isomer. The Rimmer Tiemann reaction is an exception but apparently you can
convert the o- isomer into the p-isomer. There are vast numbers of references to its preparation, including many patents. |
I don't know what you mean by "most methods" as the main two that could accomplish formylation of phenol directly are the
Reimer-Tiemann and the Duff reaction, both of which would yield salicylaldehyde. The Vilsmeier-Haack is less accessible for amateurs, and would
require the phenol to be protected in order to be employed, and unless you use a very bulky protecting group that would sterically disfavor
o-substitution, you'll probably get primarily o-formylation or a mixture with that too.
Bouveault aldehyde synthesis is another option, though for that you'd need to make p-chlorophenol, which shouldn't be too difficult, but would require
carefully controlled chlorination of phenol to ensure that you don't overchlorinate.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Check this link but it is in chinese: https://patents.google.com/patent/CN102992982B/en?q=p-hydrox...
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Sorry, this procedure used to be famous for using anethole as a precursor to mequinol, but apparently it is no longer so well-known (as the prep of
mequinol from hydroquinone has become better):
https://erowid.org/archive/rhodium/pdf/anethole.2c-b.pdf
Only the first reaction taking anethole to anisal is relevant for you, the rest are irrelevant.
Then for the demethylation it is a little harder, maybe you can use one part 48% HBr to three parts GAA, but I'm not sure if anisal will dissolve --
hopefully you can avoid gassing HBr as it is annoying... but HBr is the reagent of choice IMO for any substrate that will stand up to it, because it
is just so much easier to get/make than anything else.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
For the paper I posted, the translation says to use a reagent I, not sure if this is iodine or something else. If it is iodine, its a really
accessible synthesis.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It says right in the abstract that the reagent is a Vilsmeier reagent. See Vilsmeier-Haack reaction.
Edit: See, you should have read a little farther: "In described step (1), reagent I is POCl3 or SOCl2 or PCl5 or
PCl3" Typical Vilsmeier-Haack conditions.
If you can't even be bothered to read the paper you're asking about, this is getting into pure spoonfeeding territory.
[Edited on 10-15-2020 by Texium (zts16)]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Where was that written?
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Oh, I didn't see the translation beneath that
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
I tried to translate the paper into english using google translate, and it only gave me some parts of the paper.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Also, I knew that that was a Vilsmeier reagent, that's why I posted it.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Boffis, Texium: I am surprised you have both omitted the most famous phenol formylation, the Mg(OMe)2 method... this too is mostly
ortho of course.
The term "Vilsmeier reagent" to me always referred to the chloroiminium produced by the rxn of POCl3/SOCl2/COCl2 with DMF. Unfortunately it is very
hard to produce this kind of reagent without phosphorus compounds or SOCl2. It is higher in energy than something like Ac2O.
More generally, reactions with weak electrophiles that attack the phenolate anion, which is most of the easy methods, will usually go to the ortho
position. Reactions with strong electrophiles that attack neutral phenol will go mostly to para.
So Vilsmeier, Gattermann, formyl fluoride all go to para, while Reimer-Tiemann and Mg/HCHO go to ortho. Duff goes to ortho because of hydrogen
bonding.
The Gattermann reaction with Zn(CN)2/HCl is the most likely to be accessible to amateurs in terms of reagents, but the risks associated with improper
operation of the apparatus when you gas zinc cyanide with HCl are not appropriate for a new chemist.
The method from anethole is much safer.
[Edited on 15-10-2020 by clearly_not_atara]
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
No one has answered my question yet, is there a protecting group that will keep the aldehyde in benzaldehyde from being cannizaro-ed when it is in
molten NaOH or some other OH. I read somewhere on the forum that a much lower melting KOH and NaOH mix is possible.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
An ethylene or propylene glycol acetal might work, and is very easy to prepare.
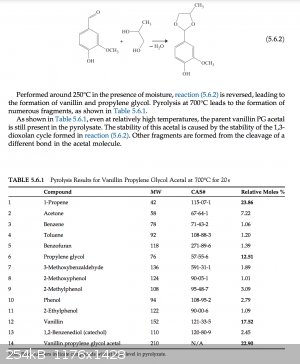
I found this excerpt from a book about the pyrolysis of various organic molecules. Acetals are well-known to be stable to strong bases, and apparently
they are pretty stable to high temperatures as well, considering this book reports heating the propylene glycol acetal of vanillin to 700ºC (!) for
20 minutes, which is a much higher temperature than you would need, and still recovering nearly 23% of the starting material. At the lower temperature
that you would be exposing it to, there should be considerably less decomposition. They do note, however, that at 250ºC in the presence of moisture,
the acetal will decompose to the aldehyde and the glycol, so you will need to make sure that your hydroxide is as fresh and dry as possible if you do
this.
Source: https://www.sciencedirect.com/science/article/pii/B978044464...
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by clearly_not_atara  | | Boffis, Texium: I am surprised you have both omitted the most famous phenol formylation, the Mg(OMe)2 method... this too is mostly
ortho of course. |
This is what I get for spending too much time in academia away from amateur chemistry.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
Does anyone know about the KOH NaOH mixture with a low melting point? Also, how do you keep moisture away from the acetal? I'm thinking that maybe
having some molten Na in the mix might destroy any water, turning it into NaOH.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Look up NaOH+KOH eutectic mixture. You do not need to keep moisture away from the acetal unless you are using it under those conditions. You can dry
NaOH and presumably KOH by drying in an oven until a constant mass is achieved, then by melting and holding above 260 C.
|
|
|
Triflic Acid
Hazard to Others
  
Posts: 486
Registered: 27-9-2020
Member Is Offline
Mood: Slowly Oxidizing into Oblivion
|
|
So, this would be the synthesis of p-hydroxybenzaldehyde,
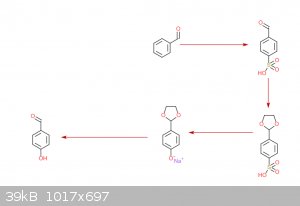
[Edited on 25-10-2020 by Triflic Acid]
|
|
|