| Pages:
1
2 |
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
I usually buy ethanol denatured with 5% diethyl ether through the pharmacy. 20 euros for a gallon.
sic transit gloria mundi
|
|
|
FranzAnton
Harmless

Posts: 45
Registered: 31-3-2020
Location: Austria
Member Is Offline
|
|
Oxidation of NH3 with Ozone
hm, the idea sounds good, because ozone can be created also from air and electricity ... but I fear that there are also a lot of side reaction? Is the
reaction save? Can the mixture of NH3 gas and O3 gas ( on top of the liquid layer) explode?
Do you have more detailed information? Yield would also interestin.
[Edited on 8-5-2020 by FranzAnton]
|
|
|
dicyanin
Hazard to Self
 
Posts: 57
Registered: 29-3-2020
Location: Europe
Member Is Offline
Mood: inquisitive
|
|
Quote: Originally posted by RogueRose  | Quote: Originally posted by Lion850  | | Science Essentials was selling 2.5 liter of 96% LR ethanol for around $70 a week ago. Maybe they still have stock. |
Man you can buy 50lbs of sugar and get about 4.5 gals of 100% ethanol for less than the $70, if you have the time. |
I just ordered some turbo-yeast, it can take up to 18% ethanol. There are instructions on the package: dissolve 8 kg sugar in 8 liter boiling water,
and dilute this to 25 liter volume. Then, after cooling to room temperature, the contents of the package are added, and after 7 days of fermenting the
result should be a solution of ethanol around 18%.
edit: maybe it's a good idea to add a few grams of diammonium phosphate as a free nitrogen source, as is generally done for wine. Maybe along with a
few grams of yeast extract and a multivitamin tablet? Probably not really necessary.
[Edited on 25-9-2020 by dicyanin]
sic transit gloria mundi
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
Purify you ethanol from car wiper fluid. At here it contains ethanol that distills over at 77-78C with short path column and MEK which seems to be get
rid of using 20g/L of NaOH upon distillation. It is excessive to make it from scratch if you aren't intending to drink it.
But, how about using ammonium sulfate and calcium nitrate to obtain ammonium nitrate? It will precipitate into calcium sulfate and what does not
settle, could be vacuum filtered to clear out? You can also use sand filter like I did to filter a really nasty, glue-like sludge. It didn't even take
that long to get 20 liters of diluted liquor through a bucket into which I made holes, put cloth, about 10cm of sand and another cloth. I presume you
use water as solvent.
[Edited on 25-9-2020 by Fyndium]
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Probably won't help you Ozzies, but Tannerite is pure, DRY NH4NO3, and at least here (Arkansas) Academy sports has shelves full
of it. As a bonus, there is a packet of Aluminum powder with it (dunno the mesh, but its fine a hell).
Now a response to the off topic thought further up:
Quote: Originally posted by dicyanin  | Quote: Originally posted by RogueRose  | Quote: Originally posted by Lion850  | | Science Essentials was selling 2.5 liter of 96% LR ethanol for around $70 a week ago. Maybe they still have stock. |
Man you can buy 50lbs of sugar and get about 4.5 gals of 100% ethanol for less than the $70, if you have the time. |
I just ordered some turbo-yeast, it can take up to 18% ethanol. There are instructions on the package: dissolve 8 kg sugar in 8 liter boiling water,
and dilute this to 25 liter volume. Then, after cooling to room temperature, the contents of the package are added, and after 7 days of fermenting the
result should be a solution of ethanol around 18%.
edit: maybe it's a good idea to add a few grams of diammonium phosphate as a free nitrogen source, as is generally done for wine. Maybe along with a
few grams of yeast extract and a multivitamin tablet? Probably not really necessary.
[Edited on 25-9-2020 by dicyanin] |
I, umm, have a bit of experience in this area (Officer, it's HAND SANITIZER, not moonshine!).
My preferred yeast is this. It needs absolutely NOTHING except sugar and water. The yeast nutrient is already in there. Half a bag (25lb) of sugar poured is a five
gallon bucket and the bucket filled about three inches from top with water. Usually use boiling water for the first gallon or two and stir to
dissolve the sugar. Check temp before pitching yeast (45C or lower). Snap on lid with fermentation lock. Sucker will be BUMPING within hours. Let
sit at least til no more bubbling through lock. Distill and keep fraction >100C. Distill again and keep fraction >82C or so. Yield about 2
litres of around 90% EtOH.
For "medicinal" purposes, the product is good first go round (will be about 120 proof, buy a hydrometer!) and is extra "medicinal" after a soak in a
jar with whisky barrel chips. I use a 2l erlenmeyer (dedicated), a claisen adapter packed with copper gauze (genuine Chore Boy, not the 3M crap, it
has some daggum coating on it) and a still head and a graham condenser. The copper catches the stinky sulfur compounds. I know the graham condenser
ain't a favorite here, but i LOVE mine.
[Edited on 9-26-2020 by arkoma]
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
symboom
International Hazard
    
Posts: 1143
Registered: 11-11-2010
Location: Wrongplanet
Member Is Offline
Mood: Doing science while it is still legal since 2010
|
|
Calcium nitrate and ammonium sulfate should work to make ammonium nitrate
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
It does, but the sludge of calcium sulphate is a pig to deal with.
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I have heard so. Apparently I have experience on similar matter as I recovered some soluble compounds from some fertilizer and filtering the sludge
was terrible using plain sand, but resulted in clear solution anyway.
|
|
|
GammaFunction
Hazard to Self
 
Posts: 78
Registered: 28-1-2013
Member Is Offline
Mood: No Mood
|
|
Why not start with bottom-shelf vodka and distill to 96% (and then sieves if needed).
In US, 1.75l of 40% vodka is as low as $13, and will distill to 0.7l of ethanol. And it's much more convenient distilling from 40% than sub-20%.
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
Quote: Originally posted by GammaFunction  |
Why not start with bottom-shelf vodka and distill to 96% (and then sieves if needed).
In US, 1.75l of 40% vodka is as low as $13, and will distill to 0.7l of ethanol. And it's much more convenient distilling from 40% than sub-20%.
|
It is a bit different here in Australia you would struggle to get a 0.7 L bottle of 40% for less than $25. For $10 I can get enough sugar to make
around 3 L of ethanol. I also enjoy and am well set up for the fermenting and distillation.
|
|
|
Mateo_swe
National Hazard
   
Posts: 548
Registered: 24-8-2019
Location: Within EU
Member Is Offline
|
|
I also have some experience making ethanol although it was some years ago now.
I also use similar turbo yeast packs, sugar and water, same procedure for the starting fermentation to around 16-18%.
I have this all stainless distill rig with built in heater that takes the whole pail about 20liter of fermented material.
On top of the boiler there is a 8-10cm diameter stainless pipe filled with rachig rings (cerapic pieces of thin tube) and at the top a thermometer and
a small tube going down to a condenser.
This device automatically dials in showing about 79°C at the top thermometer and the distilled ethanol drips out.
There are cooling water to condenser and it also goes right through the distill column at 2 places.
I get about 92% raw ethanol out that i dilute to 40% and pour it through a plastic pipe filled with activated carbon.
After the activated carbon treatment its way better than store bought vodka.
One can get it to the degree that it taste like nothing, one just feels the warmth in the chest.
One should get good quality activated stone based carbon, the better quality of activated carbon the better vodka.
If one wants ethanol for lab use do the same but dilute the carbon treated ethanol to about 20% and distill again.
How to get rid of the last % is harder, it wont go better than about 95% by distillation but i guess you all know that here.
One can use molecular sieves and some other methods to get rid of the water.
A good, well designed distill rig gets you high% ethanol on the first run but it needs the carbon treatment or maybe a second distill also works
removing the foul tasting impurities, i dont know as i use activated carbon method.
An all stainless distill rig is a bit expensive if you cant make it yourself but it will last you a lifetime.
The sad thing is my distill rig is at our summer house and havent been used for years now as vodka for drinking is less hassle to just go buy at the
store.
I have had thoughts about fire it up again just to make som absolute ethanol for experiments.
Right now all ethanol and isopropanol is bought up by people scared of the covid virus.
Maybe some isopropanol can be found at the 3D-printer stores.
They use it in large amounts to rinse the resin of 3D-printed items (the resin type 3D-printers).
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I was tinkering this because I have a bucket full of extract that I made years ago. It originates from otc lawn fertilizer that has NPK rating of
8-4-13, contains also Mg+S 2+8 and 6% of calcium. I deducted that the calcium is either in calcium ammonium nitrate, or then just plain old calcium
carbonate. So, apparently it contains no urea whatsoever, which is an excellent thing. The MSDS states that it just contains plain 25% AN and some
minor additives like copper sulfate, etc.
Thing is, I dissolved it in boiling water and filtered it through sand and recovered everything that dissolved, and dumped everything that does not.
The mixture formed literally a glue-like paste, that stuck everywhere and was literally impossible to filter with anything that resembles paper, but
it dripped through a sand filter quite decently, although I had to dilute and agitate it now and then. The resulting liquor was crystal clear, though,
so it did the job well. I concentrated the liquor until it started to mush up, and upon cooling it formed a moist white mass.
I suppose that if there were any soluble sulfates, they turned out to ammonium sulfate, which is inert to H2SO4. This could be checked by adding
magnesium sulfate (which I have at hand) and see if precipitate occurs. Dropping in carbonate might not be a good idea, because it could turn stuff
into carbonates, that react with H2SO4, expending valuable acid. I was thinking that I could do a test in tube by adding soluble sulfates and
carbonates and see if precipitation occurs in attempt to determine what it contains and in which ratio. If I had Ca nitrate at hand, I would prefer
it, because it would churn the ammonium sulfate and drop it out. Ammonium sulfate is inert to SA, so it only affects when determining the need for SA.
What would be the best net reaction to minimize the expenditure of sulfuric acid in order to extract all nitrates in form of nitric acid? I was
thinking of starting the reaction with initial charge, and dropping in sulfuric acid as it proceeds, and when no more HNO3 appears, stop addition?
[Edited on 23-10-2020 by Fyndium]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
It is important to know what exactly you're dealing with before you try to isolate and nitrate. If I understand, you have a mass of water-soluble
white solid. Why don't you try heating a small quantity to decomposition, maybe you could get an idea of actual nitrate concentration based on NOx
evolution.
It sounds like you have in solution:
Ammonium nitrate
Ammonium sulfate
Potassium sulfate
Potassium nitrate
And possibly some soluble calcium and magnesium salts. Does that sound right?
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
The MSDS states only ammonium nitrate as the ingredient at 25%, and everything else is a wild-card.
Could it be better to make a microscale distillation with H2SO4 to figure out how much HNO3 it can output? Prior, dry the ingredients to eliminate all
excess water and check the gravity of the resulting liquid. Estimating NOx fumes seems to be difficult from my point of expertise.
Anything that does not react with acid and or does not form volatiles that can affect HNO3, is ok with me.
[Edited on 23-10-2020 by Fyndium]
|
|
|
MadHatter
International Hazard
    
Posts: 1346
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Previous post
http://www.sciencemadness.org/talk/viewthread.php?tid=154741...
This is the post to the previous method to
produce ammonium nitrate from CAN and
ammonium sulphate. No longer needed for
me because I found it relatively cheap here
in the U.S. in agricultural prill form for
$2 USD/pound.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
That looks like a good post. I thought it the other way, adding calcium nitrate to crash any ammonium sulfate out. Anyway, it appears obvious that any
mixture containing calcium and sulfate in soluble form, will form an insoluble form, no matter how you put it. Both are easy to test.
The post is excellent if one wants pure ammonium nitrate. In my instance, I was only looking to extract all nitrate as nitric acid, and any
H2SO4-inert spectators shall remain, because they will be drained anyway. I initially thought as far as recovering the ammonium sulfate and finally
sodium sulfate. I don't actually have found any use for AN, because it is thermally unstable and generally any nitrate substitutes as a source of
nitrate ion. If I ever happen to need pure AN, I'll give it a look.
For my mushy mess, I recall that I filtered off a huge amount of glue-like mass, and I wouldn't be too off to say it would balance to 75% of total
volume. I remember buying two bags of 20kg of the stuff when it was on clearance sale for 70% off or something, and I ended up a bucket full of mushy
concentrate, and couple of buckets of glue-gunk. Hence the mush might already be process-ready ammonium nitrate with some minor solutes. The
fertilizer it was bought was purposes as a general garden fertilizer, not meant to be sprayed or pumped, hence it forming solids upon mixing doesn't
matter.
|
|
|
MadHatter
International Hazard
    
Posts: 1346
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
N-P-K
Here are some N-P-K values when considering
nitrogen fertilizer.
Ammonium Nitrate: 35-0-0
Ammonium Sulphate: 21.2-0-0
Calcium Nitrate(anhydrous): 17.1-0-0
CAN: 15.5-0-0
Potassium Nitrate: 13.8-0-46.5
Sodium Nitrate: 16.5-0-0
Urea: 46.6-0-0
[Edited on 2020/10/24 by MadHatter]
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
It must also be noted that packages usually state "nitrogen from urea" and "nitrogen from nitrate". So a high nitrogen content fertilizer may be far
from useful.
I also find it stupid that there are many fertilizers that contain little to almost no nitrates at all, instead they are filled with all kinds of
other things, even when sold as "general purpose fertilizer".
I actually managed to find a fertilizer sold in 10, 20 and 40kg bags for general use that consists 60% of CAN and 10% of AN, balance being probably
calcium carbonate and stuff. It is even for sale as winter is coming, so I might pick one up just for fun.
|
|
|
MadHatter
International Hazard
    
Posts: 1346
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Mixes
I agree. Match up the ingredients list
to the N-P-K rating. Unless it corresponds
to something in those ratings you could
end up with a bag of "natural" fertilizer
which could be nothing more than dried
up horse shit.
Fyndium, in your case it sounds like you
have the genuine article. Not a bad
starting point. Is it coated with
paraffin ?
[Edited on 2020/10/25 by MadHatter]
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
Fyndium
International Hazard
    
Posts: 1192
Registered: 12-7-2020
Location: Not in USA
Member Is Offline
|
|
I need to buy it first to find out what it contains. I found out that CAN is sold in two ways, either with calcium nitrate or with calcium carbonate.
If the latter, the product will be easily processed into highly pure AN. I believe although it is the second one.
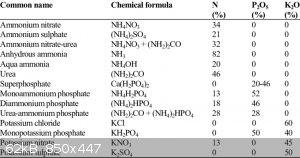
The NPK ratings tell only so much. Many compounds have nitrogen, even lots of it, but only limited few have it as nitrate. It is the
MSDS that is of a much better reference, or if the ingredients are actually listed on the product by some means.
Btw, for being bored, I purchased a 2.5kg pack of garden fertilizer for 9€. The stuff looked just as horrible as any fertilizer - like it's dug from
the corners of a piglet. MSDS stated it consisted of about 50-60% of KNO3, balance being some form of sulfate, and apparently urea phosphate - and red
dye. Fancy that it had zero solid residue - the solution was clear, although very, very dark, burgundy-colored due to the dye. It looks like the same
dye that is used for fuel oil.
I pre-washed quickly the batch with 0C cooled water with mixed ice, decanted most off and heated it to boil and added enough to dissolve all, and left
for crystallize at 0C for overnight. I decanted the water, suction filtered the crystals dry, washed with just enough of 0C water, and then with
little ethanol. Most of the color was gone, but I sort of like it how they look. I don't bother to recrystallize them second time, because I will feed
them to sulfuric acid anyways. The yield? 980 grams, or about 78% based on MSDS. From 2.5kg, it's not good to be honest. I hate it how they just fill
everything with either air, water, chaulk or something else unusable.
I found that the same stuff is sold under couple of other brands, and they state the KNO3 content between 35 to 65 percent, so I guess we're in the
ballpark.

Well, this has nothing to do with ammonium nitrate, but boy, is the KNO3 pleasure to work with, compared to the mushy sludge of the AN. I was
afraid when I saw the progress, but now I'm fascinated by the beauty of the crystals and the ease they formed - just dissolve it and wait, and you've
got pretty much pure KNO3 at hand. I think 8-year olds get excited when they do their first recrystallizations, but I still find it one of the best
things in chemistry.
[Edited on 25-10-2020 by Fyndium]
|
|
|
| Pages:
1
2 |