dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
Lead 2 acetate basic
Hello,
I want to synthese some lead diacetate by combining one mole of Lead
oxide and 2 moles of acetic acid, but was wondering, will the produced
lead 2 acetate be the basic one or the tryhidrate as i need the more
thermo stable one.
Regards.
[Edited on 14-9-2020 by dextro88]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
If you use dry reagents and don't conduct the reaction in water then the product will be dry. If your reagents aren't dry (aqueous acetic acid for
example) then you will either end up with the hydrate or a mix of the hydrates and anhydrous form depending on how much water is present. Bbuuuttttt
basic lead acetate is not the same as lead acetate hydrate. When a salt is "basic" as in basic copper carbonate or basic lead acetate, it means that
the compound is a mixed salt with at least one cation being OH- and the others being, well, anything else. This is just my basic understanding of the
concept so I'm sure other people could be more helpful. Do you want basic lead acetate?
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
thanks for your input, i already thought this,
but that mean i will need atlest some water in there to be able to donate OH atoms, but that will lead to some byproduct as the tryhidrade in the
mixture, maybe i need to try out both anhydrous procedure and a little bit wet one, and will try out a destructive decarboxilation of phenylcarboxilic
acid, and yield will tell, any more wise ideas ?
Actualy i cheked both molecules :
https://pubchem.ncbi.nlm.nih.gov/compound/Lead_II_-acetate-b...
https://pubchem.ncbi.nlm.nih.gov/compound/Lead-acetate-trihy...
and find out the tryhidrate is just with 3 OH cations, and the basic one have just 2, so that mean i will need to dilute the acetic acid with 2 moles
H2O per mole of PbO, i am right ?
Regards
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Pretty sure the net reaction goes something like that: PbO + 2AcOH --> Pb(OAc)2 + H2O. So there's probably no need for OH
ions in order for the reaction to take place.
Also note that the reaction forms water as a byproduct, so you probably won't get anhydrous product even in anhydrous conditions. Luckily,
Pb(OAc)2 is really easy to dry, just put it in the oven and wait(it won't decompose till ~176C)[1].
Lastly, as long as you use acetic acid, I don't think it's likely for any basic salts to be formed in the reacrion.
[1] - https://pubs.acs.org/doi/10.1021/acs.inorgchem.6b01116
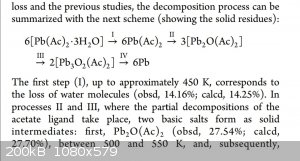
[Edited on 16-9-2020 by B.D.E]
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by dextro88  |
and find out the tryhidrate is just with 3 OH cations, and the basic one have just 2, so that mean i will need to dilute the acetic acid with 2 moles
H2O per mole of PbO, i am right ?
|
Check again. There's 3 water molecules, not 3 OH ions.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I think if you don't know the difference between hydroxide ions and water molecules, you might want to put off messing with lead salts until you've
got a better grasp of chemistry.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
dextro88
Hazard to Self
 
Posts: 60
Registered: 20-10-2018
Location: In the lab
Member Is Offline
Mood: loading...
|
|
Quote: Originally posted by B.D.E  | Quote: Originally posted by dextro88  |
and find out the tryhidrate is just with 3 OH cations, and the basic one have just 2, so that mean i will need to dilute the acetic acid with 2 moles
H2O per mole of PbO, i am right ?
|
Check again. There's 3 water molecules, not 3 OH ions.
|
actualy i dont see good on the picture and was thinking abaut these OH ions njl was talking, and posted too fast, was thinking abaut whole another way
of mechanism, where water will donate OH ions to the acetate salt and was wondering how this will happen in these acidic conditions.
But, thanks to B.D.E for the kind answer, that clarify a lot of thoughts i was having, so simply stiring the melted salt at high temperatures will
dehydrate to the basic salt as i understand.
And last, i know the toxicty of lead salts and espesily the lead diacetate, and its usage in history and and all tested toxicity, i got natural
solution for such reactions 
[Edited on 16-9-2020 by dextro88]
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
It seems like your question was answered but I have no idea what your last response means. Just make sure you wear gloves/goggles and have some water
nearby to thoroughly wash any spills.
|
|
|
yobbo II
National Hazard
   
Posts: 765
Registered: 28-3-2016
Member Is Offline
Mood: No Mood
|
|
See the 'Lead Salts preparation' thread for (perhaps more info)
|
|
|