ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
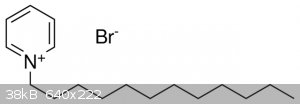
1-Dodecylpyridinium bromide as PTC
Hi!
I've been looking into some PTC catalysts for organic oxidations and reductions and have stumbled upon an OTC compound known as 1-Dodecylpyridinium
bromide (supposedly used to be in a drug called laurosept). Do you think it could be used as a phase transfer catalyst? If not, is there an OTC or
semi-otc way of obtaining TBAB or other PTCs? Here, in Poland there's only one supplier with them and they're ridicolously expensive in comparison
even to Sigma or Fisher.
Thanks for all the suggestions!
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
How about reaction of n-butyl bromide/iodide with ammonia? This should yield quartenary ammonium salt.
n-butanol should not be very hard to get, then reaction of it with HI from KI + H2SO4.
1-dodecylpyridinium bromide looks like surfactant. Not sure if PTC catalyst shoudl form micelles rather it should not form them.
[Edited on 25-7-2020 by goldberg]
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
That would yield NH3R+ Br/I- though, I think the PTCs need more than one long chain to be effective. Not sure.
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If you add enough butyl iodide you get the quaternary salt.
On a related note, the world is currently full of hand sanitisers- most seem to be based on alcohol, but some are quats like this stuff
https://en.wikipedia.org/wiki/Benzalkonium_chloride
Does anyone know (preferably from experience) if they work as PTC?
Because I can buy benzalkonium at a few % w/w by the gallon.
e.g.
https://www.wickes.co.uk/Wickes-Fungicidal-Wash---4L/p/14195...
or
https://www.accesstoretail.com/uploads/documentation/ACF25DA...
[Edited on 26-7-20 by unionised]
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Could you test some by adding some of the amine to a solution of potassium permanganate say and then adding a solvent say toluene or petroleum ether?
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
If you can get a tertiary amine and a haloalkane, you can make them pretty easily for yourself.
I made TEBAC a few times from TEA and BnCl in acetonitrile, it is really easy.
There is also a video on it out there: https://www.youtube.com/watch?v=tJZvVeiBEbs
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Judging by the smell it's got some other stuff in there that might react with permanganate too.
But I will see how it goes.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Thanks, that's an interesting suggestion!
I don't think I'll be able to replicate that though. Both triethylamine and acetonitrile are expensive here and it isn't really possible to get a hold
of small quantities easily. That's why I was wondering about some OTC PTCs, as there has only been one thread on sciencemadness that didn't spark much
ideas.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Okay, now I kinda feel dumb - there's a fantastic prep of TBAB made by Melgar.
Here it is, pasted:
One-pot, quick-and-dirty tetrabutylammonium bromide (TBAB) preparation
Lab space has recently become somewhat of a luxury for me, so I've been looking at various syntheses that don't really require chemistry glassware, or
extensive purification. TBAB seems to be one of the few things that can be made this way, since getting an exact quantity is less important than
getting the emulsion to form and dissipate like you expect it to. So from a solution, it can just slowly be added until it performs like you think it
should, assuming the solution is unreactive to your reaction conditions. That being said, here goes:
You will need:
Sodium or potassium bromide
Sodium carbonate, either solid or as a saturated solution
Sulfuric or polyphosphoric acid
n-butyl alcohol, or the industrial solvent "butyl cellosolve" (2-butoxyethanol)
Methylene chloride, or another hydrophobic solvent that is inert to strong acids and weak bases and immiscible with your acid
Any ammonium salt besides ammonium chloride, or a strong ammonium hydroxide solution
A glass bottle 100 mL or less, with a chemical resistant, airtight cap.
A pipette and a funnel
Some sort of heating device. An alcohol lamp can work.
A freezer, or cold weather.
Begin with about 1/5 the volume of your bottle of butyl alcohol, or about 1/8 its volume of 2-butoxyethanol.
Measure out 1.5 molar equivalents of bromide salt for the quantity of butyl compound you plan to use. 2-butoxyethanol requires three times as much
bromide per mole as n-butanol. Combine both in the bottle, then add roughly the same volume of nonpolar solvent as you have for your butyl compound.
Add 1.5 molar equivalents (based on sulfur or phosphorus acids) of your oxoacid, and cap it.
Let any evolved heat dissipate, then shake it well. Keep repeating this step until heat stops evolving.
If the solids aren't going into solution, cool the bottle down to freezer temperatures, then open it and add a small quantity (about 10-20% the volume
of your oxoacid) of water. There should be at least a third the volume of the bottle empty.
Cap the bottle, and carefully heat it, keeping it below water's boiling point. Depending on the nature of your reagents, the alcohol/alcohol ether
should dissolve some of your acid and salt, and the acid should dehydrate your alcohol, turning it into an alkyl bromide, which should migrate to the
nonpolar layer. Swirl the bottle occasionally, to make sure there is a thorough mixing of the reagents in the aqueous/acid layer.
I have not tried this procedure with 2-butoxyethanol, however the HBr should cleave the ether bond, and then additional HBr should brominate the
resulting hydroxyl groups. I see no reason that this wouldn't happen, but feel free to correct me if I'm wrong.
When the layers stop changing size and/or color, allow the bottle to cool, then carefully open it, wearing gloves, and with a rag or paper towel over
the cap to prevent spray. None of the contents should have an extremely high vapor pressure at these temperatures, but regardless, it's prudent to
release any gases slowly, so they don't cause the contents to foam out of the bottle.
Use a separatory funnel to remove the lower layer, and put the upper layer back into the bottle. Or pipette the lower layer into a waste container, or
do whatever works to remove most of the lower layer. You do not have to be extremely thorough here.
Add solid sodium carbonate until there's no more bubbling, then add a small quantity more, at least enough to indicate when an acid is being
neutralized.
Figure out what to do with the 1,2 dibromoethane if you used 2-butoxyethanol. I haven't tried this myself, so I can only speculate.
Add your ammonium salt or aqueous ammonia solution, dropwise. Add a few drops, then mix the solution and wait for a few minutes. Keep doing this until
the solution has an ammonia or amine odor to it that doesn't quickly subside. If at any point, the lower layer becomes acidic, add more sodium
carbonate. You should now notice the solution become a characteristic emulsion upon stirring, which separates after a minute or so.
Acidify the lower layer with a ~50% solution of your oxoacid. This will form salts with any non-quaternary amines in the upper layer, bringing them to
the lower layer.
Your upper layer should consist mostly of TBAB and whatever solvent you used. In many cases, it can be pipetted from this layer directly, and used
as-is.
Any thoughts or suggestions?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Well, the good news is that the stuff didn't immediately react with permanganate so my fear that it might have alcohol as a co solvent were unfounded
but, even in a solution saturated with salt, the stuff didn't persuade the purple colour to dissolve into the lighter fuel I used as a pet ether
substitute.
On a related note, I discovered that the last stage of the alkylation of amines has a name.
https://en.wikipedia.org/wiki/Menshutkin_reaction
Seems odd that the "earlier" stages of the alkylation don't seem to have a name.
https://en.wikipedia.org/wiki/Amine_alkylation
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Speaking of benzalkonium chloride, if I react BAC with NaOH which alkyl substituent leaves the nitrogen (assuming BAC has the structure of
dimethylbenzylamine akyl chloride)? Are the 2 methyl groups covalently bonded while the longer chain is more ionic or are all of the substituents
bonded in the same way?
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
@njl, benzalalkonium chloride is a quaternary amine salt, if you react it with NaOH you with simply produce benzalalkonium hydroxide. Sometimes on
boiling these hydroxide break down but not necessarily by an obvious route, N-alkyl heterocyclic salts being a case in point.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
That makes sense. I hadn't considered hydroxide as an anion, I just assumed the reaction would leave an alcohol and NaCl. I'm struggling to wrap my
head around why something like tetramethylammonium hydroxide doesn't immediately decompose into methanol and trimethylamine. Are the C-N bonds all
covalent? Or do they have intermediate properties between ionic and covalent bonds?
|
|
|