| Pages:
1
2 |
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
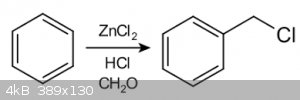
Blanc chloromethylation employing iodine
Hi,
recently I've been interested in the use of Blanc chloromethylation as a way to synthesize 2,5-dimethoxybenzaldehyde.
I was wondering if it would be possible to employ iodine in the reaction instead of chlorine. Bromine works for sure.
The reasoning behind this is that the formed byproduct - bis(iodomethyl) ether would be less volatile, therefore potentially less likely to give one
lung cancer. Also the formed iodobenzyl derivative could give higher yields with subsequent reactions, as it's a generally better leaving group.
I already asked that on one other forum, but sciencemadness has much more users.
I'd be grateful for any suggestions.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
From wikipedia: "The corresponding fluoromethylation, bromomethylation and iodomethylation reactions can also be achieved, using the appropriate
hydrohalic acid.[7] "
Following that given reference, we find this: " C., Norman, Richard O. (2017). Principles of Organic Synthesis, 3rd Edition. Coxon, James M. (3rd
ed.). Boca Raton: Routledge. ISBN 9781351421737. OCLC 1042320639."
Searching further, we can find this: http://library.sciencemadness.org/library/books/organic_reac...
Which has an example and names further references for the iodomethylation.
You could start from there on and look further for more references if you need more informations on it.
It is definitely possible, that is apparent with these information.
I find this very interesting!
Also, they name a high yield for their given example(90%), and that, together with the obviously much higher yield in the sommelet reaction(if that is
your plan) makes it look quite interesting.
The downside will then remain the cost of it....
But as for bis(chloromethyl) ether and the bromo analogue, both are in small scales, if any are even produced, still readily hydrolysed to harmless
compounds in the reaction mixture and thus are not even likely to escape the reaction in the first place.
Given that not even the bis(chloromethyl)ether is very volatile either, and has a high boiling point too.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Wow, thank you karlos!
I've been searching for it for a while, but didn't think of the most obvious one...
I thought about employing another strategy instead of the Sommelet (as it is not very high yielding and can produce impurities).
I'm attaching a very interesting paper about it - using very OTC compounds (KNO3 and KOH) paired with a PTC like CTAB to convert benzyl halides to
aldehydes in great yields! They span from 75 to 90%.
I'm also attaching a paper talking about basically the same thing (NaNO3 and NaOH) but ommiting the use of a PTC (while preserving the high, 90ish
yields). That could be potentially completely OTC.
One user also made me think of using the Kornblum Oxidation. Pretty OTC too, while maintaining high yields. Only the triethylamine remains a thing to
buy. Unless it could be substituted for sodium methoxide?
Attachment: liu2008 (1).pdf (170kB)
This file has been downloaded 457 times
Attachment: 2010-090 (2).pdf (195kB)
This file has been downloaded 474 times
[Edited on 5-9-2020 by ArbuzToWoda]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
The kornblum STINKS, I wouldn't want to use any DMSO based method ever again after my own and only experience with it.
I don't think the amine can be substituted easily there.
But for the kornblum it is possible, apparently even with NaHCO3... but under heat, some more stinky smelly Me2S...
Here: http://ursula.chem.yale.edu/~chem220/chem220js/STUDYAIDS/oxi...
On the other hand, the higher temperature makes it easier to remove the dimethyl sulfide as soon as it formed, and then bubble it into a hypochlorite
solution to neutralise its horrible stench.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Yeah, I wouldn't even consider it without a scrubber.
A bit of an offtopic here, but obtaining 1,4-dimethoxybenzene - any experiences with it? I though it'd be best to follow this procedure for
hydroquinone -> p-methoxyphenol: https://www.researchgate.net/publication/284274797_Selective...
Dimethyl sulfate is unobtainable for me, and apart from that it's awful to work with.
Then maybe use methyl iodide to extensively methylate the phenol? Or would dimethyl carbonate prove better? I've seen a writeup by Antoncho about it,
but it's really old and only an experimental section. Apart from that not much...
Attaching the paper if someone could enter the link or something
Attachment: CurrentOrganicChemistry2013171108-1113 (1).pdf (177kB)
This file has been downloaded 429 times
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Can you get trimethyl phosphate? This works very good and is cheap and nonsuspicious.
Although I never tried that or anything similar.
I rather prefer to simply buy the respective benzaldehyde.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
I suppose, but quite expensively. On the other hand, dimethyl carbonate is much cheaper and I could make methyl iodide easily.
I wish I could just buy 2,5-DMB. The thing is, I could only do it in bulk (150-1kg) and I'm not a wannabe drug dealer, so I don't need that much.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I would suggest dimethyl oxalate if you would prefer to save your iodide. It can be prepared from the obvious precursors:
https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
In the thread above some people tried using more methanol than recommended by Orgsyn with disappointing results. I think this may be because excess
methanol leads to the production of dimethyl ether and water, which inhibits the reaction. Or maybe the methanol dilutes the sulfuric acid reducing
the acidity of the mixture.
Instead methanol should probably be just a little above stoichiometric; Orgsyn uses 2.5:1 MeOH:H2C2O4:
http://www.orgsyn.org/demo.aspx?prep=CV2P0414
The formation of dimethyl oxalate is not favorable, so H2SO4 is acting as both acid catalyst and dehydrating agent. I would suggest following the OS
procedure closely, as applying the intuition developed from other esterifications may give the wrong ideas.
EDIT: actually I'd bet you could make dimethyl oxalate from oxalic acid + dimethyl carbonate with maybe just a little acid catalyst
[Edited on 5-9-2020 by clearly_not_atara]
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
I don't really mind using up methyl iodide, I'm just unsure if it would work in okay yields. I've got around 150g of iodine and it should last for
long enough, especially if I'll be recovering it from the reaction mixtures.
I've taken a look at the OrgSyn procedure and it looks promising. I only have the hydrate of oxalic acid though, and no Dean-Stark to dry it.
|
|
|
RadicallyStabilized
Harmless

Posts: 35
Registered: 3-10-2018
Member Is Offline
|
|
Quote: Originally posted by ArbuzToWoda  |
A bit of an offtopic here, but obtaining 1,4-dimethoxybenzene - any experiences with it? I though it'd be best to follow this procedure for
hydroquinone -> p-methoxyphenol |
I'm not sure if there is a good way from 1,4-dimethoxybenzene to the benzaldehyde you want. You could however do a Reimer-Tiemann formylation on
p-MeO-phenol and then O-methylate the 2-hydroxy-5-methoxy-benzaldehyde. DMC might work but is it high-yielding? Maybe somebody knows. There is however
an interesting method using Me3SBr here: https://www.sciencemadness.org/whisper/viewthread.php?tid=15...
According to Nicodem yields should be quite satisfactory and it avoids the need to work with methyl halides. Might be a bit smelly though so nothing
for karlos 
Everyday consciousness classifies and subordinates, coerces under patterns of easy manipulation and disregards the essential.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Well, swoner on TheVespiary reported using the Blanc bromo methylation to install the -CH2-Br on the carbon next to -O-CH3 and then employ the
Sommelet reaction to change it into -CHO. Here's the link: https://www.thevespiary.org/talk/index.php?topic=18076.msg54...
Does the aldehyde help with methylation? Or could one just use the Me3SBr on p-MeO-phenol?
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Does anyone know if dimethoxy benzaldehyde is as susceptible to oxidation as regular benzaldehyde?
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Doesn't seem like so - I think the methoxy groups donate a bit of the electron density making the aldehyde more stable. That's just a theory, may
someone else take voice. It's also sold with multi-year expiration dates and is a solid, all things combined it should be quite shelf-stable.
[Edited on 7-9-2020 by ArbuzToWoda]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
The aldehyde makes methylation more difficult. Methyl iodide is quite strong enough as long as an appropriate solvent is used.
I should warn you that it is not generally acceptable here to ask for open-ended advice (spoonfeeding) regarding how to produce chemicals like
2,5-dimethoxybenzaldehyde. You can discuss the chemistry of particular reactions, but SM does not allow "manufacturing advice". In particular,
questions like "which is the best route to make you-know-what" offend the conscience of some of our members and possibly the hosting company.
There are other, more appropriate places for such topics.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
I understand, atara.
I don't think I've quite asked for any spoonfeeding though? I posted many sources, asked about experimentals and proposed interesting reactions.
Neither have I asked for a route, as I already know it - just for potentially higher-yielding, while still OTCish (isn't OTCism valued a lot here?)
alternatives.
Regards.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
Arbuz that's what I was thinking. Also I agree I don't think there's any spoonfeeding here, but this is the kind of thread that could easily go that
direction 
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
My concern exactly. We started with chloromethylation, then it was O-methylation, then it was Sommelet, Kornblum, Riemer-Tiemann, боже
мой!
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
I think we're still discussing the chemistry though. Also I haven't been on this board for long but it seems like most people know better than to
humor someone who's only here for recipes.
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
Let's come back to the topic. Atara has his rights which we should respect, as he worked hard to contribute to various chemistry forums.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Next time just write "omg" so I don't have to activate the translator engine 
[Edited on 7-9-2020 by karlos³]
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 350
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by ArbuzToWoda  | | Let's come back to the topic. Atara has his rights which we should respect, as he worked hard to contribute to various chemistry forums.
|
I desagree completely @ArbuzToWoda. This chemistry Forum does not belong to @Clearly Not Atara.
SM is a website for members to exchange experiences in chemistry. It is not a website to discuss only theoretical chemistry. Not even the site
moderators themselves try to enforce rules as strict as @Clearly not Atara tries to enforce. This is a space for providing recipes, for discussing
drug synthesis, psychoactive or not, among other things. I am suspicious to speak because I have discussed several times in public with @Clearly Not
Atara here on the Forum because of this kind of authoritarianism on his part, to say the least.
If I want to learn or discuss theoretical chemistry I will do it with the professor of my college or I will instruct myself through books, never
through a forum that even by the name "ScienceMadness", implies the fact that the members who are here are or consider themselves as mad scientists,
eager for practical experiences in chemistry, either in the field of explosives, drugs, pharmaceuticals or simply fun chemistry with different
pyrotechnics, colorful or special effects.
Just the fact that someone comes here on the site to authoritatively say what the members can or cannot do or say here in this space, completely
contradicting the moderators speech really causes me disgust and I hope that the other members and the moderators vehemently disapprove of this type
of authoritarian and anti-democratic conduct.
it is better to be silent than say so much bullshit
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 350
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
And to complete my reasoning and do what I always do which is to contribute to the forum with papers and research, here are two files that instruct on
the synthesis of 2,5 dimethoxy benzaldehyde from 4-methoxy phenol.
I never waste time talking nonsense, I always present practical methods to synthesize the desired chemicals the members want.
Attachment: 2,5-Dimethoxybenzaldehyde from 1,4 dimethoxy benzene - hydroformilation with paraformaldehyde.PDF (55kB)
This file has been downloaded 460 times
Attachment: 2,5-Dimethoxybenzaldehyde from 4-methoxy phenol.pdf (72kB)
This file has been downloaded 629 times
|
|
|
ArbuzToWoda
Hazard to Self
 
Posts: 98
Registered: 15-7-2020
Member Is Offline
|
|
I understand your position, Chemi Pharma.
I think I can agree with you to a certain point. Not many good chemistry forums exist. There's the chemistry boards (filled with irritating
overly-sensitive nerds), there's TheVespiary (very lovely, engaged moderators) and there's ScienceMadness. I'm ommiting LambdaSyn and Hyperlab as
they're not really international. The thing is, all of these forums deeply discourage spoonfeeding drug chemist (all chemists for that matter, but
clandestines especially). There has been no spoonfeeding in this thread, but I suppose atara just worries about some dumb people coming and asking for
straight up recipes without any understanding of chemistry. Can't call that attitude anything near the word "science", so I wouldn't agree with that
either. We all have that fear in mind that some of these forums would go in the same way that Hive went, which is, as we know it's history, not
preferable.
Atara might've overreacted. I suppose that's because he's already had to do with people that did go too far.
Thank you for the papers! I'll take a deeper look into them.
Regards
[Edited on 8-9-2020 by ArbuzToWoda]
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
Oh how nice, thank you for that compliment 
We're doing our best 
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | Not even the site moderators themselves try to enforce rules as strict as @Clearly not Atara tries to enforce. |
I think you are responding to an overzealous interpretation of my post. I was merely suggesting the thread stay on topic. But I am
quite okay with finding that the guy who used to get mad at me for posting this stuff is now standing on the other side of the field. 
Related to the topic, the big concern with hydriodic acid in this rxn is the potential for demethylation. HI is one of the most powerful demethylating
agents. IIRC SN2 rates are much higher with I- vs Br-.
|
|
|
| Pages:
1
2 |