Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Manganese ii and iii acetates
I'll first report on manganese iii acetate as this is what I made first...unplanned.
- 20g of MnSO4.xH2O was dissolved in 70g water, giving a pale pink solution.
- 20g Ca(CH3COO)2.H2O was dissolved in 80ml water, giving a clear solution.
- Solutions were mixed under stirring.
- Solution slowly turned white as CaSO4 was formed and going into suspension. See photo. Stirring was continued for another 15 minutes.
- Stirring was stopped, and the white CaSO4 ppt settled out. But the supernatant solution was turning light brown!
- The solution was filtered and boiled down. It went dark brown quite fast. I realised it was being oxidised to Mn iii, Mn iii OAc being a stable
manganese iii compound.
- The solution was boiled down on the steam bath until a dark brown liquid remained that did not appear to be giving off any more steam.
- The crucible was removed from the steam bath and vigorously stirred as it cooled. By the time the temperature dropped to the point where it could be
held by hand the contents became a lighter brown bone dry solid.
- 25g was recovered. There is a mention online of the dihydrate being a cinnamon color, so I think that is what I ended up with: Mn(CH3COO)3.2H2O. See
photo.
- The hard bits still stuck to the crucible was dissolved very easily in hydrochloric acid, giving a green-ish solution, see photo.
Manganese ii acetate
Reading online on the crystal growing wiki "https://en.m.crystalls.info/Manganese(II)_acetate":
"Water solutions are hydrolytically unstable and rapidly oxidizes by atmospheric oxygen to brown manganese(III) acetate. So it`s recommended to add
excess of acetic acid for stopping the hydrolysis."
I decided to have another go at manganese ii acetate. This time I used the amount ratios as per the crystal wiki (scaled down). But, I do not know
what hydrate my manganese sulphate is (bought on eBay as fertilizer) and I just assumed it is the heptahydrate mentioned in the wiki.
- 14.2g calcium acetate monohydrate was dissolved in 50ml glacial acetic acid. But after a few minutes of stirring it became a solid jelly! 90ml of
water was then added which resulted in a clear solution.
- I did not want to add manganese sulphate in solution as that would result in an eventual large volume to evaporate down, and I wanted to evaporate
at room temperature. So I added manganese sulphate in small amounts direct to the stirring solution waiting a few minutes between each addition until
a total of 23g was added.
- After some 20 minutes of stirring the solution was left to settle out. The result was a white ppt of calcium sulphate with a pink supernatant
solution, as I was hoping for.
- The solution was filtered, and the white remainder washed by decantation with glacial acetic acid. The pink filtrate was placed in a crucible in the
desiccator, over NaOH and under strong vacuum; the solution started to boil at room temp under vacuum; I suspect the excess acetic acid was boiling
out from the vinegar smell.
- The white remainder was dried on a steam bath. The final weight was 15g, which was more than the expected 11g. However, while the majority of the
ppt was snow white, there was a crust of very pale pink material....
- The solution of manganese ii acetate took some 60 hours in the desiccator to dry out. After 24 hours the NaOH was completely wet and was replaced
with dry NaOH.
- Eventually after 60 hours there was a light pink hard, dry mass in the crucible. 20g was recovered which was exactly what the target was. See
photos. Initially the salt had a slight vinegar smell but after breaking it up this reduced to almost nothing.
- Cleaning the bits left in the crucible showed it to be very soluble in water.
So where did the extra weight of recovered calcium sulphate came from? I suspect my manganese sulphate is not the heptahydrate but probably one of the
more common lower hydrates. This meant that there was excess manganese sulphate, which then crashed out of solution with the lead sulphate due to the
acetic acid content of the solution. Does this sound feasible?
With hindsight I probably needed much less acetic acid to prevent hydrolysys. But it seemed to have worked.
       
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Well that is interesting, when I was making Mn III Acetate I had to oxidise Mn II acetate with KMnO4 or it can be done with electrolysis. I doubt that
your Mn III acetate is pure neither have I ever seen a preparation in such way.
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Are you sure that you made Mn(III) acetate? It's make by oxidation of Mn(II) acetate by KMnO4 in glacial acetic acid. Mn(II) acetate forms slightly
acidic solution and Mn(II) salts are stable at these conditions. I think there was partial hydrolysis of your Mn(II) acetate in to Mn(OH)2 which
oxidized in to brown MnO(OH). Probably this compound contaminate your product and caused brown coloration.
Most common hydrates of MnSO4 are mono and tetrahydrate.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks for the feedback gents! So I have a light brown solid that melts into its water of hydration (?) forming a dark brown liquid at reasonably low
temperature (I think between 40 and 50), is very soluble in water, no smell. At no point was there any sign of a ppt when I boiled it down, so all of
it seems soluble in water.
When I can get back into the shed I will try to heat it and see what happens to the dark brown liquid at higher temperature. If it is manganese
acetate it should decompose at some point. I have not managed to find the decomposition temperature online yet but will search more.
As I mentioned in the original post, it says on the crystal wiki ""Water solutions are hydrolytically unstable and rapidly oxidizes by atmospheric
oxygen to brown manganese(III) acetate" but how much authority this statement have I have no idea.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I would expect a bright pink for Mn{(iii) acetate. I would also ecpect instability with water. (This is based on vast experience of making a Mniii
salt in solution once and seeing its rapid disproportionation.)
I would recrystallise your product from glacial acetic acid if possible.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Chemplayer Manganese III Acetate
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
My MnIII acetate is orange brown microcrystalline clumpy powder and bought commecially but you need a potent oxidizing agent like KMnO4 like Bedlasky
pointed out above. What you have is almost certainly a basic MnII acetate or a mixture with Mn oxides.
Mn II formate and acetate are a delicate pale pink when I have made them (there is a thread on Mn II formate: http://www.sciencemadness.org/talk/viewthread.php?tid=76724#...)
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi j_sum1 - online (various locations) described Mn iii OAc as a dark brown solid and a cinnamon brown dihydrate. Mn ii OAc is a light pink solid.
Does anyone know the melting temperature for Mn iii OAc dihydrate and anhydrous melting and decomposition temp?
I found info for Mn ii OAc: on Wikipedia anhydrous melts at 210 °C and the tetrahydrate at 80 C. According to this post
https://www.researchgate.net/post/By_heat_treatment_of_1500_...
some decomposition of manganese ii acetate is already happening at 300C.
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by j_sum1  | | (This is based on vast experience of making a Mniii salt in solution once and seeing its rapid disproportionation.) |
Trivalent manganese can be stabilized in water by certain ligands - EDTA and phosphate (or even better pyrophosphate) works well. Fluoride should also
work, but I never try it. However fluoromanganates(III) can be prepared (according to literature) by electrolysis of MnF2 in 40%HF/KF solution - and
this have a lot of water.
Other Mn(III) salts must be prepared in solutions with a very low water content - like glacial acetic acid, concentrated sulfuric or phosphoric acids
etc.
Lion850: If you are interested - Mn(OAc)3 preparation
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by Lion850  | Thanks for the feedback gents! So I have a light brown solid that melts into its water of hydration (?) forming a dark brown liquid at reasonably low
temperature (I think between 40 and 50), is very soluble in water, no smell. At no point was there any sign of a ppt when I boiled it down, so all of
it seems soluble in water.
When I can get back into the shed I will try to heat it and see what happens to the dark brown liquid at higher temperature. If it is manganese
acetate it should decompose at some point. I have not managed to find the decomposition temperature online yet but will search more.
|
It shouldn't be that soluble.
Quote: Originally posted by Lion850  |
As I mentioned in the original post, it says on the crystal wiki ""Water solutions are hydrolytically unstable and rapidly oxidizes by atmospheric
oxygen to brown manganese(III) acetate" but how much authority this statement have I have no idea.
|
I tried making MnO2 the same way, when I saw wikipedia saying that Mn(OH)2 readily decomposes at normal air to MnO2 and I wanted to make some MnO2 by
this method as it is way easier and faster to obtain Mn(OH)2 than MnO2. My child deadass though back then that it is a super idea that will work. No
way... It was only the light brown coating after a few days, which couldn't even be compared to MnO2
[Edited on 12-6-2020 by mackolol]
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by mackolol  |
I tried making MnO2 the same way, when I saw wikipedia saying that Mn(OH)2 readily decomposes at normal air to MnO2 and I wanted to make some MnO2 by
this method as it is way easier and faster to obtain Mn(OH)2 than MnO2. My child deadass though back then that it is a super idea that will work. No
way... It was only the light brown coating after a few days, which couldn't even be compared to MnO2
[Edited on 12-6-2020 by mackolol] |
Mn(OH)2 is slowly oxidized by air only in to hydrated MnO(OH). If you want to make MnO2, you must add some oxidizing agent - for example H2O2.
|
|
|
kmno4
International Hazard
    
Posts: 1503
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: | | Mn(OH)2 is slowly oxidized by air only in to hydrated MnO(OH). If you want to make MnO2, you must add some oxidizing agent - for example H2O2.
|
You will not get MnO2 from Mn(OH)2 and H2O2.
Why ?
There is a nice experiment with 30% H2O2 and MnO2.... do not try this on scale larger than 1 drop of perhydrol 
Слава Україні !
Героям слава !
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Your manganese(II) acetate looks quite well. I have a commercial sample and it has a similar pink color. If I finely grind the manganese(II) acetate,
then it becomes nearly white.
Your supposed to be manganese(III) acetate almost certainly is something else. As others pointed out, most likely some basic manganese(III) compound,
like MnO(OH) or MnO(OAc). At pH > 7, manganese(II) is very easily oxidized by oxygen from air, resulting in te formation of basic compounds.
I once made manganese(III) in aqueous solution (at very low pH, around 0). It has a beautiful reddish/brown color.
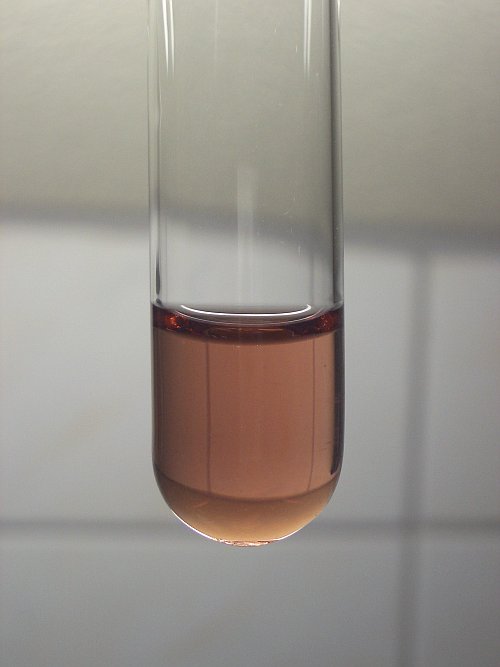
When the pH is raised (e.g. by dilution), it hydrolyses and forms a brown precipitate, most likely hydrous Mn2O3, or MnO(OH). Well below pH = 7 it
completely precipitates.

|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Quote: Originally posted by kmno4  | | Quote: | | Mn(OH)2 is slowly oxidized by air only in to hydrated MnO(OH). If you want to make MnO2, you must add some oxidizing agent - for example H2O2.
|
You will not get MnO2 from Mn(OH)2 and H2O2.
Why ?
There is a nice experiment with 30% H2O2 and MnO2.... do not try this on scale larger than 1 drop of perhydrol  |
Yes, you get MnO2 from reaction of Mn(OH)2 and H2O2.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
I made some MnCO3 today. Reacted HCl with MnO2, filtered and neutralized with Na2CO3, and blackish brown gunk resulted. It was settled multiple times
with water to yield light brown sediment in a sep funnel until final wash was made and light brown MnCO3 drained onto pan to evaporate. Next step is
acetification.
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Few thoughts on preparing manganese acetate.
I first added 2mol of GAA with 1mol of MnCO3 and volumetric equity of water and heated the mixture in water bath under reflux. The reaction was
overall very sluggish, and I ended up adding significantly more acetic acid and some water, and eventually after heating for total of 6 hours, the
effervesence was almost ceased, and the remaining solids settled easily to the bottom of the flask. The liquid was decanted, filtered and
concentrated, and finally evaporated until mushy crystals formed. It was once heated up to dissolve all, and then left to crystallize. Pink crystals
formed on the liquor, and they were at first collected. This is what it looks now.
The reaction is basically nil at ntp, no matter the concentration of AcOH. Even otc 5-10% (distilled) vinegar works very well on preparing acetates
(sodium, calcium, cobalt, manganese, lead, iron, for the start), but the solution must be brought to around 100C. Water bath works well, but just
boiling it in SS pot directly does the job as well.

The crystals seem to appear much more blatant in the picture than in reality, where they appear bright pink.
What is said in Crystal Growing Wiki about Mn III Acetate, is true. The pink acetate will turn into brown upon heating, apparently when content of
acid depletes, and upon exposure to oxygen. The batch I heated in order to remove hydrate water, turned brown.
Hence, the proper way to prepare Mn II Acetate is to concentrate the liquid, preferably protected from air, and allow it to crystallize and dry, and
when crystals start to form in a supersaturated liquid, let it cool down, collect and recrystallize if necessary, dry it in crystal form, grind and
store as pink powder.
[Edited on 12-7-2020 by Refinery]
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Refinery - as I mentioned in the original post my first attempt also turned brown, I avoided that by using some GAA as solvent when doing the
double displacement the second time. My product is light pink, different degrees of 'pinkness' may be link to the hydrate number? My reaction was all
at room temperature, not heated at any point. I like the look of the few acetate salts I have now seen; they look generally soft and fluffy (for lack
of better words) 
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
Now that I dried it completely and it cooled down and solidified, it turned out to be much less brown, and upon grinding it in mortar, it was rendered
pale pinkish powder.
Funny that when my friend walked in when I was scraping the acetate solids from the glass pan with a SS paint scraper(which is by the way a perfect
tool for scraping or chiseling anything from glass surfaces, not limiting to chemistry), he laughed and said "now we're in business" - because the
crumbled rocky pieces of pinkish-brownish pieces looked exactly the same as crack cocaine in wikimedia commons. I had to explain, for his
disappointment, that even though I measured total yield of 108 grams, which, from 57.5g of MnCO3 represents a 88% yield for tetrahydrate, which I
consider nice.
|
|
|