nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Maleic Acid by Dehydration of Malic Acid ?
I am interested in glyoxylic acid production by the ozonolysis of maleic acid.
Unfortunately, maleic acid is not readily available, but it seems convertible to malic acid and vice versa.
I have found many references on malic acid production from maleic by double hydration, but almost no references on the reverse route (dehydration of
malic to maleic).
One reference mentioned this route, however. By heating malic acid at 250 °C, it should dehydrate to maleic anhydride, which can be subsequently
hydrated to maleic acid!

Furthermore, malic acid seems to be an interesting precursor to whole host of important compounds, such as succinic, fumaric, tartaric and other
acids, their anhydrides and several solvents such as butyrolactone and THF:
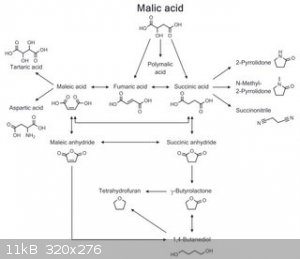
Source: Kövilein, Aline, et al. "Malic acid production from renewables: A Review." Journal of Chemical Technology & Biotechnology.
The above image does not contain direct arrow from malic to maleic, instead it shows two routes:
malic -> fumaric -> maleic
malic -> succinic -> fumaric -> maleic
I already have fumaric acid and it is easier for me to obtain than maleic, but I have to research how this can be done (seems like isomerisation from
trans to cis form). Again, I found only articles on the isomerisation of maleic to fumaric, not vice versa (fumaric is more stable configuration so
it's preferred). Perhaps it's possible only biochemically.
The only alternatives for glyoxylic acid production I found are electrolysis and oxidation of glyoxal (made by oxidation of acetaldehyde).
I don't have electrolysis equipment and the nitric acid oxidation seems hard to do properly (the reaction is strongly exothermic and overoxidation to
oxalic acid occurs easily).
But I have a good quality ozonizer, so the only problem is obtaining maleic acid.
Although I can order it directly from chem supplier, it seems to be way more expensive than malic acid. Ordering glyoxylic acid monohydrate is
possible as well, but this might be frowned upon as it is used clandestinely for the production of controlled precursor substances.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
A quick look into the literature revealed a little bit. From Wygand/Hilgetag's "Preparative Organic Chemistry":
Page 388:
| Quote: | Maleic anhydride can be obtained in 90% yield if
the water liberated is removed by azeotropic distillation with tetrachloroethane. Fumaric acid rearranges when heated, giving maleic
anhydride. |
Page 822:
| Quote: | | Dicarboxylic acids of maleic-fumaric acid type are almost always obtained from halogenated succinic acids; fumaric acid is easily formed from
monobromosuccinic acid, which loses 1 equivalent of hydrogen bromide when merely boiled with water or heated above its melting point.
|
So it seems fumaric acid might be a good source material. I have not found information about how much to heat the acid for it to rearrange
into maleic anhydride.
Making fumaric acid first from succinic/2-bromosuccinic acids seems not worth the hassle.
But still dehydration of malic acid seems to be the cheapest route to maleic anhydride... Maybe I will just give it a try using simple distillation.
Maleic anhydride has a b.p. if 202 degC, while than malic acid decomposes between 225 - 235 degC. So maybe careful distillation under reduced pressure
will first remove the water of dehydration, then the anhydride product.
The anhydride will be then rehydrated to obtain maleic acid.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Okay I've probably got it 
Malic acid are two carbons connected by single bond, having three groups (-H, -OH, -COOH):
| Code: |
HOOC COOH
\ /
H -- C -- C -- H
/ \
HO H
|
By heating, OH- and H+ are detached and leaves as H2O. Electrons move to center forming a double bond and ultimately two possible geometric
configurations:
| Code: |
HOOC COOH HOOC H
\ / \ /
C == C C == C
/ \ / \
H H H COOH
|
These are called maleic and fumaric acid, respectively.
On further heating, maleic acid readily forms anhydride.
Fumaric acid might not dehydrate so readily, but some suggest that given enough heat, it will anyway.
Fumaric acid does dehydrate to maleic anhydride when string dehydrating agent is added (PCl5). Without it, I am not sure.. maybe some conversion will
happen along with pyrolysis and formation of side products.
But since malic acid is cheap, I don't care...
You can imagine how the two carboxyl groups at cis position lose water and the molecule cyclizes to the respective anhydride.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Recently I was thinking about making fumaric acid from malic acid, which I need and unfortunately due to epidemy shipping problems is very expensive
in Poland at the time, but didn't find nothing practical in internet...
But as for glyoxylic acid synthesis I have something like that (from hive):
made it by electrolysis of oxalic acid in dilute sulfuric acid (lead electrodes) and evaporated (<40 deg C, glyoxylic acid is unstable at elevated
temperature) catholyte used directly for synthesis.
But since you don't have electrolysis equipment, doubt that it will be useful
[Edited on 6-5-2020 by mackolol]
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
You can definitely make fumaric acid from maleic (see Wikipedia: Isomerization to fumaric acid) though you might need a catalyst.
You can get malic acid cheaply from winemaking supplies (they also sell tartaric acid). It's dirt cheap - like 5 USD for a kilo. Then dehydrate it and
distill to maleic + fumaric or hopefully just maleic, then isomerize to fumaric.
Electrolysis is a no go for me at the moment as setting it up is too big of a project and I already have everything needed for ozonolysis.
I will soon try the synthesis from malic acid. Making fumaric acid is also interesting as it has several uses (e.g. purification of amines via
fumarates).
Maleic anhydride is a suprisingly versatile source material to a whole host of important organic compounds.
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
Okay I've finally found the answer in [1]. I've look in the index term "malic acid" and bingo! This comprehensive book about maleic
anhydride (MA - a super important feedstock in chemical industry) briefly discussed historical methods of production - on page 19 it says:
| Quote: | | Of course, the first preparation of MA was the dehydrative distillation of malic acid.(17) |
The reference goes to [2]. It's not the exact same article, but fortunately the author published the work in two other journals in
the same year. I have attached the articles for anyone interested.
Unfortunately my german language is virtually non-existent and the antique article is not really OCR/translator-friendly.
What I understood is that distillation of malic acid gives variety of products, one called "meta-malic" acid (maybe maleic acid?).
After all that searching I have finally resorted to just purchasing MA and prepare maleic acid from it - however if anyone is interested in making MA
from malic acid, here is one reference.
My ultimate goal is to prepare glyoxylic acid (which is very expensive), starting from malic acid, which is very cheap.
The reason I am keen on antique chemistry is that more and more chemicals are getting regulated or becoming prohibitively expensive (I am applying for
university only to have "license" for purchasing chemicals) - so finding an old method that works just fine for an amateur is sometimes golden.
[1] Trivedi, Be. Maleic anhydride. Springer Science & Business Media, 2013.
[2] J. Pelouze, Anal. Pharmacie 11, 263 (1834)
[Edited on 2-5-2023 by nimgoldman]
Attachment: phpAXSONI (173kB)
This file has been downloaded 197 times
Attachment: php3xz8S8 (589kB)
This file has been downloaded 190 times
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Three years is a long time to be dedicated to this rxn. Have you any experience with ozonolysis? That would be the more difficult part of this
process, most likely. Be absolutely sure that you don't breathe the ozone -- luckily, it has a half-life of fifteen minutes, and isn't really a
pollutant (tropospheric ozone exists in equilibrium of production/destruction via NO2 photolysis).
|
|
|
nimgoldman
Hazard to Others
  
Posts: 303
Registered: 11-6-2018
Member Is Offline
|
|
| Quote: |
Have you any experience with ozonolysis? That would be the more difficult part of this process, most likely. |
I have an ozonizer sitting on shelf for almost 4 years, unused, as I never had an opportunity to use it. I still need to put together equipment to
even start playing with it. Stuff like source of oxygen (compressed gas or a concentrator), thermos flask for dry ice bath etc. etc.
|
|
|