Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Possible routes for synthesis of esters of tertiary alcohols and phenols, using carboxylic acids
i would like to make esters from carboxylic acids since they can be oxidized from alcohols made in grignard reactions, giving many options to explore
esters from several combinations of carboxylic acids and alcohols, millions of unique smells.
Making an acid chloride
acid chlorides react with tertiary alcohols and phenols to completion to form esters.
Use thionyl chloride or phosphorus chloride
Cons: Very expensive hazmat shipping, difficult to make at home
Making acid anhydrides with ketene.
Acid anhydrides react with tertiary alcohols and phenols to completion, similarly to acid chlorides
https://www.youtube.com/watch?v=g9sPlMkYEIw
cons: Very dangerous
Steglich esterification
https://www.organic-chemistry.org/namedreactions/steglich-es...
Pros: None of the chemicals require hazmat shipping, available from chemsavers
Cost: a 100 gram bottle of N,N-dicyclohexylcarbodiimide from chemsavers is $32.70. this is 0.485 moles of DCC. 1 mole of DCC makes 1 mole of
ester, .485 moles of tert-butyl acetate is 65 mL. One 100 gram bottle can go a long way, since you don't need to make much ester if you're only
collecting them for their smells.
Making imidazoles
source: chapter 3 of "Azolides in organic synthesis and biochemistry"
uses dicyclohexyldiimidazole, which is slightly more expensive than DCC
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
You forgot the boric acid method for phenols. Posted here only 9 days ago!
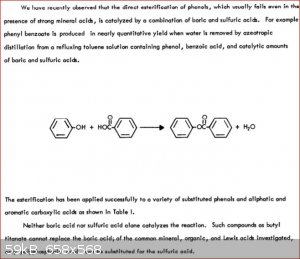
From Attachment: boric-acid-lawrence1971.pdf (69kB)
This file has been downloaded 360 times
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
he investigations of Béhal and other authors (loc. cit.) have led to the generally accepted view that the esterification of alcohols with the formic
acid/acetic anhydride reaction mixture leads to the exclusive formation of formates. A reinvestigation, however, has shown that under the conditions
used by Béhal, acetates are also produced. Generally, the amounts of acetates formed decrease in the order primary, secondary, tertiary alcohols; in
the last case acetates are formed to a negligible degree. This finding leads to a simple and convenient method for the preparation of pure tertiary
alkyl formates in high yields. The results obtained are discussed in relation to conflicting literature data as regards the synthesis of formates.
https://onlinelibrary.wiley.com/doi/epdf/10.1002/recl.196408...
while formyl chloride is unstable, i've been wondering if you could just generate formyl chloride in situ by dripping thionyl chloride into a mixture
of formic acid and alcohol, and the formyl chloride would react instantly before it decomposes.
Another method for formate esters of tertiary alcohols using boron oxide https://www.google.com/url?sa=t&source=web&rct=j&...
[Edited on 21-4-2020 by Cou]
[Edited on 22-4-2020 by Cou]
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Does this work for synthesising oxalic acid phenyl esters used for chemiluminescence? This would be very useful as it would omit the need for oxalyl
chloride. I must try this indeed.
[Edited on 21-4-2020 by mackolol]
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by mackolol  |
Does this work for synthesising oxalic acid phenyl esters used for chemiluminescence? This would be very useful as it would omit the need for oxalyl
chloride. I must try this indeed.
[Edited on 21-4-2020 by mackolol] |
I don't know if it works oxalic acid but I do know that oxalic acid is decomposed by concentrated sulphuric acid at temperatures above about 100C. Yes
it did work in reasonable time below 100C it would be very useful to generate diphenyl oxalate.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
kmno4
International Hazard
    
Posts: 1504
Registered: 1-6-2005
Location: Silly, stupid country
Member Is Offline
Mood: No Mood
|
|
| Quote: |
Does this work for synthesising oxalic acid phenyl esters used for chemiluminescence? This would be very useful as it would omit the need for oxalyl
chloride. I must try this indeed.
|
The paper (from TL) is the only one mentioning such reaction.
The authors use mysterionus catalyst, being a mixture of H2SO4 and H2BO3 of unknown composition.
Unfortunalety, heating of phenol and oxalic acid (in toluene etc) give aurin and some soluble solid only. This solid has m.p. very close to DPO, but
it is not the ester (it is organic compound of complicated structure).
ps. jak będziesz miał jakieś sukcesy, daj konicznie znać. Tę śmieciową publikację znałem od dawna, ale nie wygląda na to że to działa z
kwasem szczawiowym.
Слава Україні !
Героям слава !
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by kmno4  | | Quote: |
Does this work for synthesising oxalic acid phenyl esters used for chemiluminescence? This would be very useful as it would omit the need for oxalyl
chloride. I must try this indeed.
|
The paper (from TL) is the only one mentioning such reaction.
The authors use mysterionus catalyst, being a mixture of H2SO4 and H2BO3 of unknown composition.
Unfortunalety, heating of phenol and oxalic acid (in toluene etc) give aurin and some soluble solid only. This solid has m.p. very close to DPO, but
it is not the ester (it is organic compound of complicated structure).
ps. jak będziesz miał jakieś sukcesy, daj konicznie znać. Tę śmieciową publikację znałem od dawna, ale nie wygląda na to że to działa z
kwasem szczawiowym. |
I didn't know about the synthesis of aurin, but it seems like this is gonna be main product. Maybe I'll try this in future, maybe substituted phenols
such as trichlorophenol behave differently in the synthesis. I'll let you all know when I will perform such synthesis.
p.s. Dzięki za słowa wsparcia, miło usłyszeć ojczysty język na zagranicznym forum 
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by kmno4  | | Quote: |
Does this work for synthesising oxalic acid phenyl esters used for chemiluminescence? This would be very useful as it would omit the need for oxalyl
chloride. I must try this indeed.
|
The paper (from TL) is the only one mentioning such reaction.
The authors use mysterionus catalyst, being a mixture of H2SO4 and H2BO3 of unknown composition.
Unfortunalety, heating of phenol and oxalic acid (in toluene etc) give aurin and some soluble solid only. This solid has m.p. very close to DPO, but
it is not the ester (it is organic compound of complicated structure).
|
Perhaps it was wishful thinking the same author has patent on the method.
But I note that the aurin synthesis requires about 140C and 24 hours so perhaps the aurin would only be a minor product below 100C.
PS: I just checked the reaction time in the boric acid paper. Its 8 hours at about 75C the toluene/water azeotropic boiling point. I also noticed
the catalyst is "1 - 5mol % boric acid and sulphuric acid" which I interperate as 1-5 mol% of boric acid and 1 -5 mol% of sulphuric acid.
I suspect the aurin synthesis requires the oxalic acid to decompose to react with the phenol.
[Edited on 4/22/2020 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
And how much is 1-5mol%? I don't understand such value as mol%...
[Edited on 22-4-2020 by mackolol]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
5 mol% would be a ratio of 1 mole acid to 20 moles reactant
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Ok thanks, for sure will be useful, especially in my high school 
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Which one would you guys recommend for making esters such as tert-butyl acetate?
Acid chlorides are difficult to get b/c of hazmat shipping. Acid chlorides can be made from carboxylic acids, but each method has disadvantages too.
Thionyl chloride and phophorus trichloride have hazmat shipping. Triphenylphosphine is expensive.
I'm leaning towards the Steglish esterification. It's still expensive (triphenylphosphine and DCC are similar prices by weight), but none of the
chemicals require hazmat shipping, and a step is eliminated (no need to convert carboxylic acid to acid chloride first)
i hope i can make the ester of triphenylmethanol and acetic acid one day.
[Edited on 6-6-2020 by Cou]
|
|
|
Fery
International Hazard
    
Posts: 1026
Registered: 27-8-2019
Location: Czechoslovakia
Member Is Offline
|
|
Hi Cou, just an idea:
t-butanol + HCl -> t-butyl chloride (this works well, I did it already)
t-butyl chloride + Na acetate -> t-butyl acetate (did not try yet)
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
This could work good, I made benzyl acetate this way.
But with tert-butyl halides I think there is an increased risk of elimination instead of substitution.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by karlos³  |
This could work good, I made benzyl acetate this way.
But with tert-butyl halides I think there is an increased risk of elimination instead of substitution. |
Oooohhh....do you have a write-up for this?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
No I am sorry I do not.
But it involved refluxing in ethanol for 1-2h or so, and was followed by an extraction after the ester was precipitated with water(into DCM).
Those are the only details I can give you, it is quite a few years ago no and I never regarded that as an important experiment, I was just curious
about the smell of benzyl acetate.
I don't think that heating would be a good idea in case of the reaction of tert-butyl halides with a carboxylate salt, due to the elimination.
|
|
|