| Pages:
1
2 |
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Oxygen generator designs?
I do quite a bit of metalworking and glassworking that sometimes requires oxygen gas. Bottled oxygen is pretty expensive, especially since I haven't
had the budget to invest in a proper sized oxygen tank, but instead I use single use 800 mL bottles of it. They last about 10 minutes and cost $30, so
it gets expensive fast.
I'm thinking what with oxygen being literally everywhere, and being a reactive element, it shouldn't be too difficult to make a device which can
release oxygen gas upon activating it, and then recharge using the oxygen in the air.
A big requirement is that it would need no other input than air and energy. I guess water or something is ok too, but you get the point. Having to
refill it with a chemical makes it no better than buying oxygen.
I intend to eventually get myself an oxygen concentrator, the molecular sieve/zeolith kind, but I can't afford one right now.
I have built a decent capacity water electrolysis unit(1.5 kW) which would provide enough oxygen, but the design having very small gaps between plates
will immediately mix hydrogen and oxygen. If someone has an idea of how one could scrub hydrogen from the gas without exotic materials, that would
work...
I know something like an "oxygen battery" can be made with potassium permanganate, but I haven't been able to find a local source for it.
Are there more options available?
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Potassium permanganate is expensive, so that's no option either.
I've been looking into this too, but I need a (new) lathe in order to build equipment, so I've been stuck for a while now.
My plan is to make an electrolyser using a membrane to keep oxygen and hydrogen separated. I have two cylindrical SS electrodes. Using water with some
soda lye will give great conductivity and a good yield. The charge density is said to need remain relatively small so that good bubbles are formed
instead of a whitish foam. From calculations my device is rated at ~120A, but this may need to be taken down considerably in order to get bubbling
instead of foaming. I can't test for any real results, because I can't complete my device.
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Bezaleel  | Potassium permanganate is expensive, so that's no option either.
I've been looking into this too, but I need a (new) lathe in order to build equipment, so I've been stuck for a while now.
My plan is to make an electrolyser using a membrane to keep oxygen and hydrogen separated. I have two cylindrical SS electrodes. Using water with some
soda lye will give great conductivity and a good yield. The charge density is said to need remain relatively small so that good bubbles are formed
instead of a whitish foam. From calculations my device is rated at ~120A, but this may need to be taken down considerably in order to get bubbling
instead of foaming. I can't test for any real results, because I can't complete my device. |
I've considered building a membrane separated electrolysis cell too. Don't you think two electrodes is a bit too few though? That would force you to
push 120 A at only 2 Volts, so you'd have significant losses unless you use gargantuan cables...
What's your plan for the oxygen? 250 ish watts of electrolysis isn't going to sustain a significant flame, but if it's for other purposes then I guess
you kay not need quite the same volume.
In my electrolysis unit I have 15 plates(30 ish volts) * 100 cm2 ish and run it between 50-100 A, allowing me to run it from my DC welder. This
provides a needle sharp, quite small but unbelievably hot flame.
My unit is, according to most "HHO calculators" out there, rated to 15 amps, but I've done as much as 100 A in it just fine. It foams, but that's not
necessarily a problem. The foam is caught and separated in a chamber, and then rerouted back to the cell once it's liquid again.
If I were to build a separated electrolysis cell, I think I would do a series cell configuration. I'd 3d print the plate separators in nylon, and
allow for a membrane to be mounted there.
Speaking of which, what do you have in mind for the membrane? I was thinking nylon cloth, or maybe fibreglass cloth, but I suspect nylon would hold up
better what with lye dissolving glass in high enough concentration...
I have tonnes of lathes, so maybe I'd want to have a go at your design... But what is the lathe needed for?
[Edited on 3-4-2020 by Junk_Enginerd]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
What you want is pressure-swing adsorption. Oxygen can be concentrated using zeolites.
Unfortunately due to COVID, these machines will be harder to get due to increased demand and reduced supply.
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Metacelsus  | What you want is pressure-swing adsorption. Oxygen can be concentrated using zeolites.
Unfortunately due to COVID, these machines will be harder to get due to increased demand and reduced supply. |
I know, as I mentioned in my OP that's my long term goal. Unlimited oxygen from little electricity. For my needs it doesn't even matter if it's 100%
oxygen; 90% oxygen is just 10% worse. But right now I don't want to spend the cash for one, plus as you say it'll be hard to get one in the next few
months, especially a cheap chinese one which is what would give me most bang for the buck...
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Junk_Enginerd  | I do quite a bit of metalworking and glassworking that sometimes requires oxygen gas. Bottled oxygen is pretty expensive, especially since I haven't
had the budget to invest in a proper sized oxygen tank, but instead I use single use 800 mL bottles of it. They last about 10 minutes and cost $30, so
it gets expensive fast.
I'm thinking what with oxygen being literally everywhere, and being a reactive element, it shouldn't be too difficult to make a device which can
release oxygen gas upon activating it, and then recharge using the oxygen in the air.
A big requirement is that it would need no other input than air and energy. I guess water or something is ok too, but you get the point. Having to
refill it with a chemical makes it no better than buying oxygen.
I intend to eventually get myself an oxygen concentrator, the molecular sieve/zeolith kind, but I can't afford one right now.
I have built a decent capacity water electrolysis unit(1.5 kW) which would provide enough oxygen, but the design having very small gaps between plates
will immediately mix hydrogen and oxygen. If someone has an idea of how one could scrub hydrogen from the gas without exotic materials, that would
work...
I know something like an "oxygen battery" can be made with potassium permanganate, but I haven't been able to find a local source for it.
Are there more options available? |
If you are really interested in doing a high output O2 generator, LMK. I designed on about 2 years ago. It's basically the same things medical
patients use but is capable of delivering 20-50L/min (depending on how you scale it & the compressor you use). It's actually pretty simple in
what you need to do it, pressure sensors, solenoids, air filtration/dryers, zeolite holding tanks, regulators and an air compressor (even a crappy oil
filled compressor can be used though not ideally - you just need to filter the air with something like activated carbon & maybe something else).
As long as it doesn't need to be medical grade O2, you can make something pretty easily and use something like Arduino's, raspberry pi's or PLC's to
control the device. I suspect you can get 90-95% very easily at the above volume, but if you need 98%+, the volume is going to drop fairly quick.
I'd like to work on this project, there are some online groups that have tried, but they never seemed too dedicated and they were trying to make
somethign they could sell, so they were talking $10-20K for a unit, which is ridiculously high for what is needed (if you build it yourself).
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Yeah, again, that's the long term idea. I'm a mechatronics engineer so that part of it is certainly no issue, and I'd gladly build one, except I can't
for the life of me find a good source of the zeolite I'd need. Other than that design of the unit itself seems dead simple as far as I can tell. No, I
certainly won't need >95% concentration. Shit I'd probably be happy with 70% since that's still waay more heat in a torch than air gets you. As
long as the other 30% is nitrogen or other mostly inert gasses, and not hydrogen as in my electrolysis cell lol. That'll turn any piece of steel into
a hydrogen embrittled sponge in no time unfortunately.
$10k-$20k? What the actual fuck? It's literally just a damn low performance compressor, some ceramic rocks and basic electronics! Even if it was
certified for professional medical use anything more than $2k is ridiculous, and that's my professional product development engineer opinion... I'm
100% sure I could make one for <$100.
What I have found though, is eBay O2 concentrators from china. The units in the $150 class are specified to deliver between 5-10 L per minute. Now I'm
not a moron so I'll multiply that by the China factor and be happy if it delivers a third of that, but that should still be enough for the small torch
I use most of the time. Should it not be enough it doesn't really matter, since I'll need to mildly pressurize the gas regardless(to use it with the
torch) and certainly won't use it constantly, so a short period of accumulation is fine.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
based only on what I have learned from one particular type of oxygen concentrator;
. the air must be well filtered and dried before it can 'poison' the zeolite
. the oxygen enriched air is bubbled through water before delivery to the patient
to prevent the very dry gas from dehydrating the airways and lungs
. I actually believe the published flowrates are close to reality
- I'm expecting a new unit to arrive sometime soon (now going through local Customs office) so I will measure the flowrate FYI
. I doubt that you can make a reliable and safe oxygen concentrator for the cost of a Chinese unit
(GBP259 with international shipping)
. your development of a usable product will probably be ongoing as the pandemic ends
______________________________________________________________
If someone can propose a low-tech method of estimating the oxygen concentration I will attempt it.
I am separated from my chemistry stuff so it will have to be based on commonly available items.
______________________________________________________________
here I have an analytical balance, water pump, air pump, various tubings and containers, pH papers, 34% HCl, 50% H2O2, sodium
bicarbonate, vinegar, sugar, salt and pepper etc.
anything else will need to come from a supermarket or pharmacy, or by post.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Sulaiman  | based only on what I have learned from one particular type of oxygen concentrator;
. the air must be well filtered and dried before it can 'poison' the zeolite
. the oxygen enriched air is bubbled through water before delivery to the patient
to prevent the very dry gas from dehydrating the airways and lungs
. I actually believe the published flowrates are close to reality
- I'm expecting a new unit to arrive sometime soon (now going through local Customs office) so I will measure the flowrate FYI
. I doubt that you can make a reliable and safe oxygen concentrator for the cost of a Chinese unit
(GBP259 with international shipping)
. your development of a usable product will probably be ongoing as the pandemic ends
______________________________________________________________
If someone can propose a low-tech method of estimating the oxygen concentration I will attempt it.
I am separated from my chemistry stuff so it will have to be based on commonly available items.
______________________________________________________________
here I have an analytical balance, water pump, air pump, various tubings and containers, pH papers, 34% HCl, 50% H2O2, sodium
bicarbonate, vinegar, sugar, salt and pepper etc.
anything else will need to come from a supermarket or pharmacy, or by post. |
Compressor, $30. Pressure vessels <$10, just random junk needed, it's pretty mild pressures. Bit of electronics <$30, some electromechanical
valves <$20 or build from scratch. That's most of what I'd need, except for the zeolite. What I have found seems to be between $50-$100 for the
appropriate amount. I don't think it's a problem at all to build one for <$150.
Regardless if I were to build one, I wouldn't be doing it to save money or time, I'd be doing it for the engineering kick and for higher performance.
The operating principle allows for pretty easy and cost effective scaling to basically any amount of oxygen.
Measuring oxygen... The easiest way I can think of is to burn it, but I'm by no means very experienced in chemistry. Fill a known volume container,
place some alcohol or similar fuel in there, see how much of it ends up being combusted by weighing before and after, then refer to how much oxygen
that fuel and amount needs. Though I don't know how safe it would be to ignite a volatile fuel in pure oxygen. You'd need to figure out something that
burns slow enough to not be a hazard. Maybe just a lump of charcoal or something like it?
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by Junk_Enginerd  |
Compressor, $30. Pressure vessels <$10, just random junk needed, it's pretty mild pressures. Bit of electronics <$30, some electromechanical
valves <$20 or build from scratch. That's most of what I'd need, except for the zeolite. What I have found seems to be between $50-$100 for the
appropriate amount. I don't think it's a problem at all to build one for <$150 ... |
I do not believe you !
Prove me wrong and I will gladly apologise.
______________________________________________________________
"Measuring oxygen... The easiest way I can think of is to burn it, but I'm by no means very experienced in chemistry. Fill a known volume container,
place some alcohol or similar fuel in there, see how much of it ends up being combusted by weighing before and after, then refer to how much oxygen
that fuel and amount needs. Though I don't know how safe it would be to ignite a volatile fuel in pure oxygen. You'd need to figure out something that
burns slow enough to not be a hazard. Maybe just a lump of charcoal or something like it?"
Good idea, BUT :
if carbon is burned then each molecule (or mole) of O2 burned will produce one molecule (or mole) of CO2
- so the gas volume will not change*.
If a hydrocarbon is burned then water vapour will also be produced giving a transient gas volume increase
(two H2O molecules per O2 molecule)
*except that CO2 is more water soluble than oxygen, so a little will go into solution,
and the burning will temporarily increase the temperature of the gas hence its volume.
I had considered burning a metal such as magnesium as MgO would not remain gaseous,
but I would not be sure of complete consumption of the oxygen.
I could use some iron wool and wait for it to rust, but I'm not that patient.
any more ideas, anyone ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
teodor
National Hazard
   
Posts: 923
Registered: 28-6-2019
Location: Netherlands
Member Is Online
|
|
I would use NaClO3 + BaO2 mix as in an air crafts emergency system. I believe that playing with other catalysts (like transition metals) can change
the rate of O2 production. I think this system would be capable to produce the pressure you need but of course it requires recovery of NaClO3 by
electrolysis, so I think it is best when you need short periods of high pressure few times per day.
[Edited on 6-4-2020 by teodor]
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
in airplanes they use KClO3 to make O2. when mix with iron power it will generate enough heat to drive the decomposition of KClO3 to KCl and O2. but
i don't know the ratio,but may depend on heat insulation of the container and the room temperature.this is the cheapest so far.
"A mind is a terrible thing to lose"-Meisner
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Answering one by one:
Quote: Originally posted by Junk_Enginerd  |
I've considered building a membrane separated electrolysis cell too. Don't you think two electrodes is a bit too few though? That would force you to
push 120 A at only 2 Volts, so you'd have significant losses unless you use gargantuan cables...
[Edited on 3-4-2020 by Junk_Enginerd] |
It depends on the surface of the electrodes, obviously. I based my electrode size on a small setup which ran successfully. But as I said, I never got
to testing my full scale setup. So maybe you're right, and I completely misjudged the electrode size.
Quote: Originally posted by Junk_Enginerd  |
What's your plan for the oxygen? 250 ish watts of electrolysis isn't going to sustain a significant flame, but if it's for other purposes then I guess
you kay not need quite the same volume.
[Edited on 3-4-2020 by Junk_Enginerd] |
I'm more interested in the hydrogen. But thinking about the design, I thought the oxygen might be handy as well, so I made a design having two
outlets, one for each gas.
Quote: Originally posted by Junk_Enginerd  |
In my electrolysis unit I have 15 plates(30 ish volts) * 100 cm2 ish and run it between 50-100 A, allowing me to run it from my DC welder. This
provides a needle sharp, quite small but unbelievably hot flame.
[Edited on 3-4-2020 by Junk_Enginerd] |
I'm using a welder also. It can deliver up to 150A continuously, and I wish to stay below that. The electrodes to be used are concentric cylinders.
Quote: Originally posted by Junk_Enginerd  |
My unit is, according to most "HHO calculators" out there, rated to 15 amps, but I've done as much as 100 A in it just fine. It foams, but that's not
necessarily a problem. The foam is caught and separated in a chamber, and then rerouted back to the cell once it's liquid again.
[Edited on 3-4-2020 by Junk_Enginerd] |
That feels like a good approach. I'm in doubt whether you get any separation of O2 and H2 if you produce foam though. I don't know, but it could be
that when you produce foam, it will mean that both gases are formed throughout the liquid, instead of only at the respective node surfaces.
I intended to use some large pore sintered glass fritte to catch any "aero"soles, but that assumes that decent bubbles will form, not foam.
Quote: Originally posted by Junk_Enginerd  |
If I were to build a separated electrolysis cell, I think I would do a series cell configuration. I'd 3d print the plate separators in nylon, and
allow for a membrane to be mounted there.
[Edited on 3-4-2020 by Junk_Enginerd] |
I don't have any cheap access to 3D printing, which makes the manufacture of such stuff quite labour intensive. But sure, if you can, then that's
great!
Quote: Originally posted by Junk_Enginerd  |
Speaking of which, what do you have in mind for the membrane? I was thinking nylon cloth, or maybe fibreglass cloth, but I suspect nylon would hold up
better what with lye dissolving glass in high enough concentration...
[Edited on 3-4-2020 by Junk_Enginerd] |
I took the pouch-like membranes from a lead acid battery, cut them open and salvaged those that were in good shape. These are designed to be membrane,
so I guess they should be more or less fit for the purpose. I hope they survive the intended current.
Quote: Originally posted by Junk_Enginerd  |
I have tonnes of lathes, so maybe I'd want to have a go at your design... But what is the lathe needed for?
[Edited on 3-4-2020 by Junk_Enginerd] |
The idea is to have the cylindrical electrodes stick out through the roof of the container. They will be glued in place with some polyester or epoxy,
whichever holds best. I need the membrane to be between the electrodes, so the idea is to cut a sloth in the roof plate in a lathe. I considered
milling it out manually, but the sloth I need is very small, around 1.5mm wide, and circular, lying exactly between both electrodes. (Let me see
whether I can attach some pictures later on.)
Ultimately I'd like to use a voltage source instead of a current source, so that as gas is produced and builds up between the electrodes, the surface
area that is in the liquid is decreasing, thereby progressively decreasing gas production. The membrane pore size is so small that I estimate
diffusion of O2 and H2 to be minimal. The solution pressed out of the device would go into a small expansion vessel. That way it would be semi-self
controlled.
Initially, I considered a mechanical balancing mechanism so that always equal amounts of O2 and 2 X H2 will be delivered/vented, but later I dropped
the idea, seeing that some control logic would do a much better job. I never got to the point of control logic though. I don't have any Arduino
experience, but it shouldn't be too hard for someone who has fiddled around with electronics before and who also has some programming skills (I hope).
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Sulaiman, a measured amount of air can be pulled into a large syringe. Some very small amount of concentrated sodium hydroxide
solution can be sucked into the syringe and shaken to remove any carbon dioxide from the gas if present. Next, the gas can be shaken with water and
then some acidic cuprous chloride to absorb the oxygen. It may take a few minutes to absorb it all. What is left should be nitrogen, argon, etc.
By keeping track of how much gas is absorbed, it should be fairly easy to determine oxygen concentration to a rough degree.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I just tried to find some CuCl online ... no joy.
With my presently available chemicals and equipment I can not think of a practicable synthesis method.
(e.g. no set up to safely generate and handle chlorine gas or control high temperatures.
I can go 'ghetto' but in this case I think that it is not worth the risks)
Any suggestions for a synthesis path or an alternative ?
If not I'll try rusting some iron wool.
P.S. I had a look at some relevant YT videos ... school sylabus experiments using a candle ... how dumb are science teachers ?
[Edited on 7-4-2020 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
I checked the Internet, and was a bit surprised to see so many obtuse and convoluted preparations for such a simple reagent. No, there is no chlorine
gas or high temperatures required. Basically you need HCl solution, some hydrogen peroxide, some copper metal (preferably fine-stranded copper wire),
and a glass bottle with a good seal for storage.
Copper metal is stirred with HCl solution (with a stir bar on a stir plate) and hydrogen peroxide is added gradually to dissolve the copper. Add
excess copper and a slight excess of HCl, so that hydrogen peroxide is the limiting reagent. Some heating will speed this up, and will help remove
excess water if your peroxide is only the 3% variety. I don't suggest heating if you're using the 10-30% peroxide, as the reaction will heat itself.
Once you have an appreciable amount of copper dissolved into solution, make sure the pH is still low, that you maintain an excess of copper metal in
the solution, and then evaporate down the solution with heat to concentrate it. The idea for this is to make sure that all of the residual peroxide
has been reacted. At this point you have mostly CuCl2. By pouring the solution into the bottle and adding a large wad of excess copper
wire, this will reduce to CuCl over time...faster with stirring though. If there isn't enough HCl in solution then you may notice some white crystals
of CuCl knocking about the bottom of the bottle. This is fine, but they can be dissolved by adding more HCl.
Or, you can just buy CuCl2 and stir it with copper wire and some HCl, and skip a few steps.
Anyway, the bottle has to be kept sealed, as it will remove oxygen from the atmosphere and reoxidize to CuCl2. The solution can be
regenerated as needed by adding more copper metal and HCl.
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by WGTR  | I checked the Internet, and was a bit surprised to see so many obtuse and convoluted preparations for such a simple reagent. No, there is no chlorine
gas or high temperatures required. Basically you need HCl solution, some hydrogen peroxide, some copper metal (preferably fine-stranded copper wire),
and a glass bottle with a good seal for storage.
Copper metal is stirred with HCl solution (with a stir bar on a stir plate) and hydrogen peroxide is added gradually to dissolve the copper. Add
excess copper and a slight excess of HCl, so that hydrogen peroxide is the limiting reagent. Some heating will speed this up, and will help remove
excess water if your peroxide is only the 3% variety. I don't suggest heating if you're using the 10-30% peroxide, as the reaction will heat itself.
Once you have an appreciable amount of copper dissolved into solution, make sure the pH is still low, that you maintain an excess of copper metal in
the solution, and then evaporate down the solution with heat to concentrate it. The idea for this is to make sure that all of the residual peroxide
has been reacted. At this point you have mostly CuCl2. By pouring the solution into the bottle and adding a large wad of excess copper
wire, this will reduce to CuCl over time...faster with stirring though. If there isn't enough HCl in solution then you may notice some white crystals
of CuCl knocking about the bottom of the bottle. This is fine, but they can be dissolved by adding more HCl.
Or, you can just buy CuCl2 and stir it with copper wire and some HCl, and skip a few steps.
Anyway, the bottle has to be kept sealed, as it will remove oxygen from the atmosphere and reoxidize to CuCl2. The solution can be
regenerated as needed by adding more copper metal and HCl. |
Any nitrate salt works too, right? Might be easier to find than hydrogen peroxide. It's a little messier, generates a lot of NO2 gas and introduces
other salts, but it does produce copper chloride.
I know for me at least it's absolutely hopeless to find anything other than 3% solutions of H2O2, and the other 97% is soap, moisturizers and god
knows what else. Useless as a reagent at any rate...
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Excellent
Sounds like a plan
I have approximately 34% HCl, and 50% H2O2 (no problem OTC here)
and I'm sure that I can find some copper here.
The oxygen concentrator is somewhere in transit so I have time to prepare the CuCl.
(off to bed now ... I'll work out the stoichiometry and start tomorrow)
Thanks.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
Start small; you don't need much at all, maybe 10-50ml of concentrated CuCl. I tried removing the oxygen from air in a capped syringe again with this
solution just for fun, and it does work. It took around 20-30 minutes of periodically shaking the syringe though. I got exactly a 20% loss of
volume.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Are any of the steps of the preparation significantly exothermic ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
If you pour 30% HCl into 50% H2O2, and then drop a pile of copper metal into the solution you will probably have a volcano. I 've never handled 50%
peroxide before. The 30% stuff is already quite concentrated, and I've found 10% peroxide to be very aggressive and gassy when combined with 30% HCl.
I used to use this mix when etching copper circuit boards long ago. I'd suggest starting with a beaker half full of water, insert the copper, and
then start adding the acid and peroxide in small amounts until the copper begins to dissolve.
This is definitely a fume hood/outdoor activity since some gasses can be produced.
Here's a link:
https://www.youtube.com/watch?v=PlIZs2uOmus
[Edited on 20-04-08 by WGTR]
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Thanks WGTR ... very helpful
The oxygen concentrator has just arrived.
I will test the litres per minute output and %O2 as soon as I can.
_____________________________________________________________
the only large bottles (2x 5l and 2x 9.5l) that I have are currently full of sugarwashes that have almost completed fermentation,
I hope to distill off a couple of litres of 70% EtOH for hand sanitization when a 34/35 to 24/29 adapter arrives (in transit)
I am still planning the O2 concentration test(s) ... iron and/or CuCl
P.S. for general information,
here is a photo of the page in the manual that gives an overview of the principles of operation
(click on it to enlarge it)
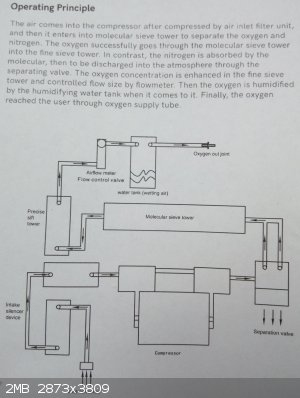
A quick YT video showing oxygen concentration https://www.youtube.com/watch?v=-z_SlavCY8M
[Edited on 8-4-2020 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Junk_Enginerd
Hazard to Others
  
Posts: 251
Registered: 26-5-2019
Location: Sweden
Member Is Offline
|
|
Quote: Originally posted by Bezaleel  |
That feels like a good approach. I'm in doubt whether you get any separation of O2 and H2 if you produce foam though. I don't know, but it could be
that when you produce foam, it will mean that both gases are formed throughout the liquid, instead of only at the respective node surfaces.
I intended to use some large pore sintered glass fritte to catch any "aero"soles, but that assumes that decent bubbles will form, not foam.
|
Sure. Again, my cell isn't designed to allow for any separation, so it doesn't matter in that case.
Quote: Originally posted by Bezaleel  |
I don't have any cheap access to 3D printing, which makes the manufacture of such stuff quite labour intensive. But sure, if you can, then that's
great!
|
Well, since so many people these days have 3d printers at home, ordering such parts is usually quite cheap, so there's always that to consider should
you want to go that route.
Quote: Originally posted by Bezaleel  |
I took the pouch-like membranes from a lead acid battery, cut them open and salvaged those that were in good shape. These are designed to be membrane,
so I guess they should be more or less fit for the purpose. I hope they survive the intended current.
|
Ah yes that's a good repurposing. However, I think those are made of fibreglass, and as such they may be degraded by sodium hydroxide, if that's your
electrolyte. I suppose it's dependent on what concentration the hydroxide is, but I don't know the details. You should probably consider using H2SO4
as the electrolyte instead of hydroxide.
Quote: Originally posted by Bezaleel  |
The idea is to have the cylindrical electrodes stick out through the roof of the container. They will be glued in place with some polyester or epoxy,
whichever holds best. I need the membrane to be between the electrodes, so the idea is to cut a sloth in the roof plate in a lathe. I considered
milling it out manually, but the sloth I need is very small, around 1.5mm wide, and circular, lying exactly between both electrodes. (Let me see
whether I can attach some pictures later on.)
|
Hmm yeah I think I'd need a picture to understand it properly...
Quote: Originally posted by Bezaleel  |
Ultimately I'd like to use a voltage source instead of a current source, so that as gas is produced and builds up between the electrodes, the surface
area that is in the liquid is decreasing, thereby progressively decreasing gas production. The membrane pore size is so small that I estimate
diffusion of O2 and H2 to be minimal. The solution pressed out of the device would go into a small expansion vessel. That way it would be semi-self
controlled.
|
Ah okay yeah, you want to avoid the "runaway" reaction that may happen as the current density would naturally increase over time with a current
source... Though in my experience, the water is consumed so very slowly that it's barely a consideration.
A big headache for me that I haven't analyzed in full yet, is whether the H2 and O2 side will be making different volumes of gas. A membrane becomes
useless pretty fast if there's a pressure differential across it. It might be necessary to regulate the pressure on the H2 and O2 side respectively,
to ensure there's no difference.
[Edited on 9-4-2020 by Junk_Enginerd]
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
An oxygen concentrator and oxygen pressuriser video with some gas torch info
https://www.youtube.com/watch?v=GxclrM270gA
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Twospoons
International Hazard
    
Posts: 1326
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
Quote: Originally posted by Junk_Enginerd  |
A big headache for me that I haven't analyzed in full yet, is whether the H2 and O2 side will be making different volumes of gas.
[Edited on 9-4-2020 by Junk_Enginerd] |
The answer to that is most definitely 'yes'. You get twice as much hydrogen as oxygen.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|
| Pages:
1
2 |
|