numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
A convenient home synthesis of brightly colored pyrylium compounds
Labs are shut down, so I find myself having a bit more free time.....
I recently got interested in these pyrylium salts and I think several of these are easily accessible to home chemistry. This actually started as a
hunt to access azulenes, but I was surprised how pretty these compounds themselves are! Only mentioned in SM as potential reagents to make amines
leaving groups, I think the compounds themselves are quite nice, and worth posting about. Many are even used as photocatalysts.
The first synthesis is that of the trimethylpyrylium tetrafluoroborate which can be used to make trimethylazulene:
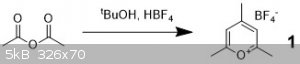
I followed a simple prep1 and adapted it here:
Acetic anhydride (13 equiv) and t-butanol (1 equiv) were added to a RBF and aqueous Fluoroboric acid (48% w/v, 0.95 equiv) was added dropwise. The
reaction was left to stir for an hour and then cooled in ice. Cold ether was added (about 2x as much as the reaction mix) and the precipitate was
collected and washed with cold ether. The product is a colorless chaulky white solid. I did this using about 4 g of alcohol and my yield was about 50%
which is substantially lower than the 70% in the paper. I think doing this under Ar and rinsing with colder ether might result in better yields. But
honestly the starting materials are so cheap… who cares.
Far more interesting are the aryl substituted pyryliums. They are substantially easier to make, not requiring Flouroboric acid. I made several of
these and I followed the same general prep for all of them. The prep is adapted from a literature procedure.2 Here is the
prep for the naphthalene and anthracene decorated ones:
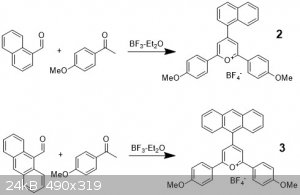
The respective aryl aldehyde (1 equiv) and acetophenone (2 equiv) were weighed into a 20 mL vial with a stirbar. If both are solids, add a little
toluene just to get things dissolved. Here I have naphthaldehyde and anthraldehyde, after which I added acetanisole which is a very common
acetophenone.

I then purged the vials with Ar and added BF3 etherate (2.5 equiv). I think you can probably get away without Ar if you do this on a larger
scale, but expect a loss in yield. The color change is immediate, going to various colors. Some turned blue, green, red, black, but is never
indicative of the final compound color. The solution is then heated to 90 oC and stirred for 2 hours.

When complete, the reactions are cooled and ether (2x reaction amount) is added. This precipitates a dark solid which is filtered and rinsed with
ether. The solid is usually dark brown or black and must be recrystallized. This is fortunately very easy to do. The solid is dissolved in a minimal
amount of boiling acetone and then cooled to 0 oC overnight. I stuck it in my freezer. Then ether is slowly added, usually about 25-50% of the amount
of acetone works well, and the solution cooled for a day further. Beautiful microcrystals with a much “brighter” color are received. The yields
are low, generally 20-50% but that might just be because I’m doing them on a small scale (<200 mg aldehyde).
I also made the following 4 compounds wanting to see some trends. They were made in the same way using respective starting materials.
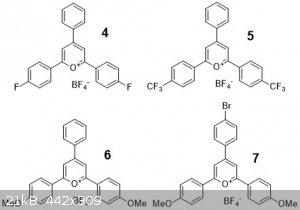
Here I have the compounds laid out, the solids, solution in acetone, and the same solutions under longwave UV. The order in all three pics is
4, 5, 6, 2, 3, 7. Note that 3
is a purple solid, hard to see in the pic.
  
Both 4 and 5 have electron withdrawing groups and similar colors, but 4 is much brighter and
fluorescent. Despite CF3 groups being more electron withdrawing, I think the single Fluorine can donate its lone pairs, which in turn
causes a “push-pull” between the electrophilic pyrylium ring and the F. CF3 groups are electronically isolated and you cannot draw such
a resonance structure.
The trend between 6, 2, 3 is also interesting as you can see how conjugation affects the color.
Compound 2 is unpublished (yay!) but I see no reason to doubt that I made it, at least for home chemistry purposes, especially since
both 6 and 3 matched literature descriptions. All three have electron rich methoxy groups that can donate
electrons, but 3 has a blue shifted absorbance (red soln instead of yellow) and red shifted emission (red fluorescence instead of
yellow) indicating a much larger stokes shift than the other two. And let me say the yellow fluorescence is REALLY bright. Don't have a UV/VIS so I
can't quantify anything.
Compound 7 surprised me. I initially made a lot more of this compound (> 1g) and installed the bromine as a synthetic handle to
maybe do Suzuki couplings or other reactions. I thought the presence of bromine would quench fluorescence as it usually facilitates intersystem
crossing to the triplet state, but maybe the “action” is far enough away from that bromine where it’s not affected.
Either way, was a fun project, I'm using a few of these to make azulenes, and although that's not working as great, I'll post the results later today.
I heard getting BF3 etherate is doable as a home chemist although I have no idea where you'd look... at least it's pretty cheap. I don't
have acetophenone, and that would have been a really nice control experiment to do with benzaldehyde. Maybe someone here can follow up on that 
1 Organic Letters, 2015 17(2) 254-257
2 Organic Letters, 2019 21(17) 7179-7183
[Edited on 4-2-2020 by numos]
|
|
|
Dr.Bob
International Hazard
    
Posts: 2757
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Great photos and pretty colors. Nice work. This is pretty interesting, I was not familair with that area of dyes.
|
|
|
Keras
National Hazard
   
Posts: 938
Registered: 20-8-2018
Location: (48, 2)
Member Is Online
|
|
Impressive and nice.
I have HF and boric acid, so I guess I could even attempt the first synthesis… When we’re free to move!
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Numos, some more really interesting chemistry and beautiful colours too! I have managed to get BF3 recently but not through official channels.
Its nice to see someone interested in chemistry beyond that of drugs.
Do you think this reaction would work with building blocks that contain simple unprotected OH or ester groups like 2-hydroxyacetophenone or vanillin
for the aldehyde? Also could you use heterocyclic substituted aldehydes/methyl ketone such as 2 or 3-pyridine carboxaldehyde or would these react
preferencially with the BF3 to form pyridinium salts?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Very cool stuff!
| Quote: | | They are substantially easier to make, not requiring Flouroboric acid. |
What role does HBF4 play in the reaction? Could you perhaps use HB(C2O4)2? ("bisoxalatoboric acid" or "hydrogen bisoxalatoborate")
https://patentimages.storage.googleapis.com/e6/37/a4/5faa017...
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
I'm glad this post was so well recieved!
Quote: Originally posted by Boffis  |
Do you think this reaction would work with building blocks that contain simple unprotected OH or ester groups like 2-hydroxyacetophenone or vanillin
for the aldehyde? Also could you use heterocyclic substituted aldehydes/methyl ketone such as 2 or 3-pyridine carboxaldehyde or would these react
preferencially with the BF3 to form pyridinium salts? |
Using vanillin is a really clever idea, I can't find any references for anyone trying it specifically, but there are definitely cases where
unprotected alcohols work to form these salts so I am confident you would get at least some conversion. I'm much less confident about the pyridines, I
think they form pretty stable complexes with BF3 and the few references that have made N-heterocycle containing pyrylium salts have done so using a
different, more complicated route.
It's an interesting thought to use other counterions, the only other examples I've seen are the perchlorate (explosive, stay away!!) and the PF6,
which is even harder to access. You need a strong acid to drive this reaction and a relatively inert "spectator" anion. As the pyrylium salt is highly
electrophillic, you want a very very weak base to be the anion.
That being said, I've never heard about this oxalatoboric acid, but it's a really cool compound, definitely have to make some! I'm not sure if it
would work for these pyrylium salts (says it's a mild acid?), but maybe it would behave similarly to perchlorate. So who knows, this is one you have
to try and let me know about! 
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Very pretty! This makes me miss working on fluorophores (I'm a biochem guy now).
The structures seem similar to anthocyanins, which can be used for dye-sensitized solar cells. I wonder how well these would work for that.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi numos, could you post those papers you referenced, maybe in the reference section of the form please?
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Nice work, thanks for sharing
Greulich, T. W., Daniliuc, C. G., & Studer, A. (2015). N -Aminopyridinium Salts as Precursors for N-Centered Radicals – Direct Amidation of
Arenes and Heteroarenes. Organic Letters, 17(2), 254–257. https://doi.org/10.1021/ol503338b
Attachment: N-Aminopyridinium Salts as Precursors for N-Centered Radicals.pdf (620kB)
This file has been downloaded 537 times
Shen, J., Li, N., Yu, Y., & Ma, C. (2019). Visible-Light-Induced Oxidation/[3 + 2] Cycloaddition/Oxidative Aromatization to Construct Benzo[ a
]carbazoles from 1,2,3,4-Tetrahydronaphthalene and Arylhydrazine Hydrochlorides. Organic Letters, 21(17), 7179–7183. https://doi.org/10.1021/acs.orglett.9b02939
Attachment: Construct Benzo[a]carbazoles from 1,2,3,4- Tetrahydronaphthalene and Arylhydrazine Hydrochlorides.pdf (1.1MB)
This file has been downloaded 442 times
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Mayko took care of the papers (thanks!), but if it's the preps you are interested in, they can be found in the SI section of both papers.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
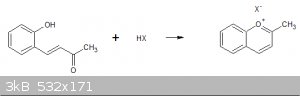
Hi Numos, do you think it would be possible to produce benzopyrlium salts from by cyclotising (2-hydroxybenzal)-acetone?
I have found that when salicylaldehyde is condensed with acetone the pale greenish product turned red at extreme pHs.
|
|
|
|