Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Neodymium iodide synthesis - powder supplied also contains Praseodymium?
I received my neodymium fine powder from China a few months ago and eventually got round to trying to make the iodide. Things did not go as expected,
most like due to the powder being contaminated.
The reaction was supposed to be:
2Nd (4g) + 3I2 (11g) = NdI3 (15g).
Water to be used as the reaction solvent.
- 100ml water with 1g Nd powder in beaker on hot plate stirrer. Stir, no heat. Grey solution due to fine metal swirling around.
- Add 2g I2. Just stir, no heat.
- After 5 minutes the solution turned olive green. Which made me excited as some suppliers mentions NdI3 as green crystals.
- After 4 minutes add 1.4g Nd (total now 2.4) and 4g I2 (total now 6).
- At 2 minutes add 2.6g Nd (total now 5) to make sure slight excess and thus consume all the iodine. Solution unexpectedly turned dark brown in a
matter of a minute.
- Add 5g I2 (total 8).
- After 10 minutes add 3.7g I2 (total 11.7) and 1.2g Nd (total now 6.2).
Final totals: Nd 6.2g, I2 11.7g. Thus Nd in excess.
- At the 30 minute mark, start to heat. Solution stays dark brown but offgassing iodine fumes when hot.
- Transfer to big beaker and 400ml water (in case low solubility of something was an issue although this was not expected).
- Stir another 3 hours. Stop for the night (no stirring) to see if there is a ppt in the morning.
- Next morning brown solution and brown ppt. Stir and heat again to boiling but no further iodine offgassing.
- Add 1g iodine to confirm offgassing starts again - it did.
- Increase water to 500 and boil strongly for an hour. After this no more iodine offgassing.
- Filer hot. Boil down to 150ml. Filter. Boil down to 50. Filter.
- Left with light brown-gold solution with a hint of lavender.
- Heat the filtrate in steam bath for hours until a crust with very little liquid. Into the desiccator under vacuum overnight.
- Gather together all the brown ppt and dry overnight.
Next day: Dry brown ppt crystals = 6.5g. The Iodide crystals dried nicely in the desiccator and yield was 11g. Color a mix of brown and yellow with
some lavender (not so clear on the photo).
But what did I have? The brown ppt made no sense. Time to do some tests.
The "neodymium" powder as received sticks to a magnet. The brown ppt does not. Neodymium (and other rare earths) are indeed magnetic.
- The iodide is very hygroscopic. A weak solution in water is very pale yellow with a hint of lavender. It gives bright yellow PbI2 with Pb(NO3)2.
With NaOH a it gives a lavender-whitish ppt. So this is looking promising for being neodymium iodide.
- The brown ppt seems very stable. Insoluble in water. It gravity filters out quite quickly (unlike precipitated iron hydroxide which is very fine).
- The "neodymium" powder dissolves easily in concentrated HCl to give a green solution that slowly turns yellow. In dilute HCl the solution is yellow
straight away when the metal powder is dissolved. Yellow should not be the color for neodymium so this is probably due to the presence of another
metal.
- The brown ppt dissolves easily in dilute HCl giving a yellow-gold solution. This solution in turn gives a very dark brown ppt with NaOH, still brown
but almost looks black. Probably the same ppt as the original ppt.
Assuming there is mostly neodymium in the bottle I received but it is mixed with another rare earth, for now I can only see praseodymium to fit the
bill because of the dark brown insoluble ppt and yellow solutions. And green initially with the strong HCl.
Could it be that for some reason the neodymium preferentially reacted with the iodine, forming NdI3 which in turn when dissolved in water give the
lavender ppt with NaOH? And the praseodymium, maybe after activation by the iodine, reacted with water to form the brown oxide as ppt?
Any ideas will be very welcome! Photos attached in sequence, I hope the file name comes up when a mouse is over each photo.
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I woulkd reclaim the money from that seller. Most likely your "neodymium" is a mix of metals, including iron.
It is magnetic, mostly due to the presence of the iron. That also explains the yellow/brown color.
Praseodymium also can be present, but praseodymium is very pale green in solution and its solids also are pale green. A mix of the (also fairly weak)
lavender color of neodymium ions and the pale green will look greyish.
Your brown and turbid solution and the brown precipitate can easily be explained by the presence of iron. Iron(III) quickly hydrolyses in aqueous
solution, giving brown/yellow and turbid solutions. On standing you get a precipitate of hydrous Fe(OH)3/FeO(OH). With iodine/iodide you get an
equilibrium between iron(II)/iron(III). This also explains the brown color and the off-gassing of iodine on heating.
Pure NdI3.xH2O has an antique pink color.
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Hi Woelen - no chance to get a refund, ordered on eBay and it was here 2 month before I started using it. So too late to open a dispute. And by the
way it said on the original listing 99.9% pure....
My first thought was also iron. But I am not sure there is a significant amount of iron. I have reacted iron filings and iodine in water a few times
and visually it proceeds differently. Once reacted and filtered iron and iodine gives a green solution that slowly change to yellow and then a dirty
brown. I never got anything else on the filter paper but leftover unreacted iron filings. Never a dark brown ppt. Although, this powder is much finer
than my iron filings. Which may cause different results. Also, it is a struggle to get iron iodide out of water by heating the solution in air. The
few times I tried I ended up with "spoiled" black stuff (that still contained a lot of iodide nevertheless). This suspect NdI3 - or whatever mix it is
- seems to be much more stable in terms of driving off the water. More like the erbium iodide was that I did a while ago.
I will keep reading and think of more tests I can do, maybe firstly on the brown ppt.
|
|
|
crown
Harmless

Posts: 3
Registered: 23-2-2014
Location: Belgium
Member Is Offline
Mood: No Mood
|
|
False Neodymium
Hi,
Two Neodymium metal powder commands from Ebay China and two false item...
Lanthane metal powder XRF certified insteade Neodymium metal power 
|
|
|
woelen
Super Administrator
        
Posts: 8014
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
@Lion850: That eBay seller is a bad seller. Selling an item as 99.9% pure, while you have such crap results with it. This, however, unfortunately is a
well-known issue with several Chinese sellers (not with all of them, there are good ones as well).
Just to be sure, if you have a thiocyanate salt, mix that with some of your solutions. A resulting red color is an almost 100% sure indication of the
presence of iron. The thiocyanate test for iron is very sensitive. Another very good test for iron is adding a slightly acidified solution of
potassium hexacyanoferrate(II) (yellow prussiate of potash). Even tiny amounts of iron give a bright blue coloration of the liquid.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
You can attempt to separate the salts through precipitation from a chloride solution as double salts of the sulfate. Add non aqueous sodium or
potassium sulfate the the neodymium chloride and collect the precipitate. I don’t remember the next step, but I think you just have to dissolve the
double salt in a hydroxide solution and keep the salt that isn't digested. Maybe someone else will remember.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
When I made this from magnets, I precipitated the rare earth with oxalic acid. Iron oxalate will stay in solution. If you calcine the RE oxalate, and
the colour is brown, then you will have Pr mixed in with you Nd. Calcination of pure Nd oxalate yields a bluish oxide.
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
Quote: Originally posted by Bezaleel  | | When I made this from magnets, I precipitated the rare earth with oxalic acid. Iron oxalate will stay in solution. If you calcine the RE oxalate, and
the colour is brown, then you will have Pr mixed in with you Nd. Calcination of pure Nd oxalate yields a bluish oxide. |
Be careful while calcining, if you heat it too intensely, you are never going to be able to dissolve it again.
List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
Thanks to all the suggestions. Poormanschemist suggested acetic acid may also be worth a try: if iron can get to iron (iii) acetate it should ppt out.
I did not have a lot of free time the last 2 days but tried with both oxalic acid and acetic acid.
Acetic acid:
- 60ml glacial acetic acid
- 5g powder
- Stir and heat
- Solution initially silver-grey from metal but quickly turned purple
- Soon afterwards turned brown
- Stir vigorously to get air to mix in plus bubble in air
- Very dark brown. Stop after another 30 minutes of stirring.
- Filter twice. Dark wine-red solution (see photo) and very dark brown ppt on filter paper. With NaOH this red solution gives a dark grey ppt. All
metal powder seems to have been consumed.
- The filtrate was boiled down and is now drying under vacuum in the desiccator overnight. But it appears to be very dark purple-red almost black, I
have no idea what this is.
Oxalic acid:
- 25g oxalic acid in 100ml water.
- 5g powder
- Some bubbles initially but then nothing.
- Add 5ml 6% H2O2
- Stir and heat a bit. Stop every so often to check color. Steadily becoming greener.
- Filer a bit of the solution and check with NaOH. Pale grey ppt - see photo.
- Stir another hour - green solution and still appears to be lots of metal left. But the metal is not sticking to the stir as easily as before - maybe
because wet. Iron filings whether wet or dry is much more difficult to remove from the stir bar.
- Stirring will continue overnight.
Gents please tell me what you think and what I should be doing different. With the amounts of acid I used I though the oxalic acid was more in excess
than the acetic acid but the reaction in the acetic acid proceeded to the point of consuming all the powder quite fast. I had some trouble to get
equations into the app I use for stoichiometry maybe I messed up.
The supplier in China said he would contact his supplier and get back to me.....let's see.
  
|
|
|
Lion850
National Hazard
   
Posts: 517
Registered: 7-10-2019
Location: Australia
Member Is Offline
Mood: Great
|
|
The supplier eventually said the powder should be neodymium, but maybe not at the published purity. So I wanted to have another go at extracting
neodymium. I decided to try and make use of the fact that the solubility of neodymium sulfate in water drops off dramatically as the water approached
100C.
I dissolved 15g powder in 15ml of 98% H2SO4 diluted with 15ml water. The powder quickly reacted and formed a grey ppt. I then added water 50ml at a
time with stirring in-between until all the ppt dissolved. This resulted in a 400ml solution with a blackish lavender solution.
This solution was filtered, yielding a clear lavender filtrate. There was some black powder remaining on the filter paper, but not much. The filtrate
was heated to boiling and boiled down. A copious amount of lavender ppt quickly fell out and by the time the volume was reduced (by boiling) to 300ml
I abandoned more boiling as the bumping became excessive (and I was in a hurry.....)
I filtered the near boiling solution under vacuum, and washed the remainder 3 times with boiling water to get rid of excess acid. The remainder
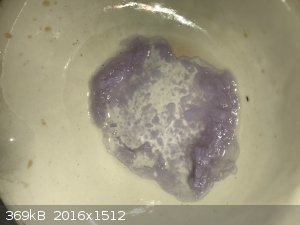
started forming beautiful lavender crystals while it was still in the filter. It was then further dried under a steel dish in the sun. This gave hard
dry lavender crystals. See photo. These crystals looks exactly like the neodymium sulfate I made from the oxide I purchased, so I was reasonably happy
I managed to get good sulfate from the powder.
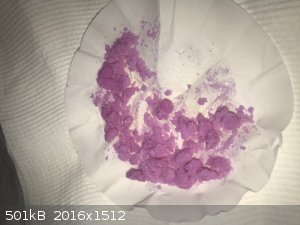
I then used a bit of the sulfate to make neodymium oxalate (double displacement with potassium oxalate) and neodymium carbonate (double displacement
with ammonium carbonate). Both are pale purple powders, see photo but the photo does not do the color justice. It's more purple to the eye.
The only test I did with the oxalate and carbonate was to test how they react with concentrated hydrochloric acid = the oxalate does nothing, and the
carbonate reacts vigorously with lots of bubbles and dissolves into a lavender solution.
I cannot find much info on neodymium carbonate, other than some supplier offers. If anyone can point me towards online info about the carbonate I will
appreciate it.
|
|
|
ChemTalk
Hazard to Self
 
Posts: 65
Registered: 13-12-2018
Location: United States
Member Is Offline
Mood: colloidal
|
|
Quote: Originally posted by Lion850  |
I cannot find much info on neodymium carbonate, other than some supplier offers. If anyone can point me towards online info about the carbonate I will
appreciate it.
|
Sorry, I think this thread is from a few months ago.. but what would you like to know about neodymium carbonate? we recently made several neodymium
compounds, including the nitrate, carbonate, phosphate, molybdate, fluoride, tungstate, sulfide, ferrocyanide and chromate.
The most interesting thing about neodymium compounds, is that they look lilac / purple in sunlight, and clear or yellow in fluorescent light.
|
|
|