goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
Voltammetric analysis of alkenes
I did not managed to find subforum related to analytical chemistry and this thread is about analysis of organic compounds so i'm posting it here. If
this is wrong, then sorry.
To my surprise i did not found any thread about amperometry here.
I'm curious if it would be possible to determine concentration of alkene by voltamerometry.
This technique looks nice to amateur due to lack of very sophisticated setup.
Building simple potentiostat looks like just two or three operational amplifiers (dirt cheap)
and piece of microcontroller with DAC and ADC to send data to computer.
Alkenes can be easily oxidized (classic detection with potassium permanganate).
So maybe it would be possible to oxidize them on anode and measure this current to determine concentration? Cathodic reduction of them while in theory
possible seems to be harder...
Does anybody came across such way of determining alkene concentration?
I could just add measured amount of stadarized amount of KMnO4 solution and then measure light absorbance but it will require significant amount of
manual labour + there are a lot of other functional groups that are readily oxidized by potassium permanganate and they will often interfere.
Or maybe coluometry? It is nice because it is an absolute method so in theory we do not have to make calibration curve.
If someone has practical experience with electroanalysis of other compounds i'm glad to hear them experience too.
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Cyclic voltammetry is a really useful tool and I think you could use it as you imagine...
But, for organic compounds it's worth considering the products of your ec reaction: you need a specific fixed electrode area to get meaningful
results. At uni I was doing CV's on organometalic complexes and it's fairly labour intensive as you need to keep grinding (to a well polished surface)
to remove the layer of polymer crap that pasivates the surface. We were using glassy carbon, it would be nice to use graphite but i doubt it would
work as liquid can soak in.
I would really like to see your setup if you get it working.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I ran across this Arduino-based DIY potentiostat that might be a good place to start.
Li, Y. C., Melenbrink, E. L., Cordonier, G. J., Boggs, C., Khan, A., Isaac, M. K., … Mallouk, T. E. (2018). An Easily Fabricated Low-Cost
Potentiostat Coupled with User-Friendly Software for Introducing Students to Electrochemical Reactions and Electroanalytical Techniques
[Product-review]. Journal of Chemical Education, 95(9), 1658–1661. https://doi.org/10.1021/acs.jchemed.8b00340
Attachment: Li et al - 2018 .pdf (3.3MB)
This file has been downloaded 342 times
Attachment: Li et al - 2018 SI.pdf (2.6MB)
This file has been downloaded 438 times
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
@mayko: Thank You for this paper didn't read it fully but at first glance it looks very usefull.
@Swinfi2: How about cleaning electrodes by applying negative pulse after analysis like in pulsed amperometric detector used in HPLC? I was thinking
about copper electrodes in form of printed circuit board (FR4 coated with copper and etching).
I found some paper in which amines were analysed on copper electrodes with good result.
Have You any idea about conditions (pH etc) for analysis of alkenes?
Also how about providing reference voltage with zener diode instead of electrochemical electrode?
I heard that electrochemical reference elctrodes are used mainly due to tradition and wide acceptance.
On sciencemadness there is paper about portable HPLC with amperometric detector and in this paper there are just three metal (gold coated) electrodes.
I'm thinking about miniaturizing setup to minimize reagent consumption with perspective for using this in FIA or low performance LC.
That's why i'm thinking about tiny electrodes apart clack of glassy carbon.
Is there any table with potentials at which given functional groups undergo reaction?
I'm also curious about using cyclic voltamamperometry for proving/disproving presence of electroactive functional groups to confirm structure of given
compound. I know that NMR is much better for this but i do not have an access to one, this same with IR and MS.
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Using negative to clean the electrode would only work if the reaction is reversible which some are, it kinda depends on your experience with the
specific system so you don't exceed the stability window as thst will lead to mixed junk that will need mechanical cleaning and screw up the data on
the reverse potential sweep.
As long as the electrode is stable it can be used but I think you'd be better plating gold onto copper as it's more inert and easier to get hold of
than platinum.
That said it's not particularly easy to guess what the best electrode would be because things like platinum were too catalytic and so promoted
decomposition with what we were doing. I can't comment on reference electrode as we always used Ag/AgCl.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
I use lots of blood glucose test strips. The Mylife ones (see pic below) have two small electrodes that look gold plated. You can just see them inside
the thermometer shaped area inside the brown area. Contact is made on the reverse side by the meter. I often wounder what use I could put the used
ones to.

Below is a pic of a test strip with the cover removed to show the gold coloured electrodes. There is a also a pic of a different brand of test strip
again with the cover removed showing the printed electrodes.

The gold coloured electrodes are about 1mm in diameter.
[Edited on 1/26/2020 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
@wg48temp9: Glucose strips are quite complex. They are using enzyme that converts glucose to hydrogen peroxide and then hydrogen peroxide oxidize gold
electrodes and this current is measured. It would be interesting to try what else this enzyme can convert to H2O2 but this reaction can be rather
selective due to enzyme nature.
Anyway i'm curious how manufacturer solved issue with reference electrode. Here we do not see any classical electrochemical electrode and there is no
place for it on a strip.
Also how about using Pb/PbSO4 electrode? I do not have any pure silver and i do not have idea where i could buy small amount of it. Piece of silver
wire is not a big amount so a price would be also affordable but i did not found a place where i can buy just a few cm piece of wire.
Also any experience with coluometry? I'm interested in this technique because it is an absolute method and i do not have any other way to prepare
standards to calibrate non-absolute method. I'm thinking about preparative TLC to get pure compound, scraping it off the plate and then coluometry.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
@goldberg: My understanding is the test strip contains glucose oxidase which oxidises the glucose to an acid which increases the conductivity of the
blood which is measured by the meter. So the two electrodes are part of a conductivity meter. No voltage reference electrode is required.
I have purchased several cm of silver wire from a silversmith at a jewellery repair shop. He was happy to give me the wire for free but I insisted I
pay him for it, just in case I need more. I don't know if the wire was an alloy or not.
No I have no experience of coluometry but as a an electronics engineer I have always been interested in it.
I spent some time in a lab that that did a lot of electrophoresis using slabs of gels to separate and isolate proteins. Apparently capillary
electrophoresis is a modern analytical tool. Which appears relatively simple to construct. Yes it not coluometry but it does use electricity and
electrodes LOL.
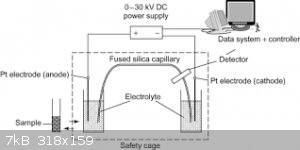
PS: sterling silver wire is sold on ebay and also pure silver electrical wire or what is claimed to be pure silver. Silver plated copper wire is
common.
But why use a silver or lead reference electrode? Why not use readily available copper electrode see
https://en.wikipedia.org/wiki/Copper%E2%80%93copper(II)_sulfate_electrode
[Edited on 1/27/2020 by wg48temp9]
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|
goldberg
Hazard to Self
 
Posts: 90
Registered: 28-4-2018
Member Is Offline
Mood: No Mood
|
|
@wg48temp9: thanks i will check my local jewellery shops. What should be purity and diameter of silver wire?
I was thinking about Pb electrode because in case of copper electrode i have solution containing copper ions and i'm not sure if they will interfere
with analysis after getting through electrolytic key.
Maybe it should be separate topic but if You mentioned capillary electrophoresis another similar technique is micellar electrokinetic chromatography.
Unfortunately i did not managed to find anything to coat capillary with to make it less brittle than plain glass capillary.
|
|
|
SWIM
National Hazard
   
Posts: 970
Registered: 3-9-2017
Member Is Offline
|
|
I know very little about this subject, but I do have a few grams of 70% gold 30% palladium wire sitting around.
It is 1/8 mm in diameter.
Would something like that be a possibility or would the palladium content be problem for catalytic reasons?
Is the diameter reasonable?
I have no idea if using really thin wire (or palladium/gold alloys)would be a good or bad idea, but I've sure got it, and as far as I know its not
easy to get in small quantities so I'm just letting you know it is available.
|
|
|
wg48temp9
National Hazard
   
Posts: 786
Registered: 30-12-2018
Location: not so United Kingdom
Member Is Offline
|
|
Quote: Originally posted by goldberg  | @wg48temp9: thanks i will check my local jewellery shops. What should be purity and diameter of silver wire?
I was thinking about Pb electrode because in case of copper electrode i have solution containing copper ions and i'm not sure if they will interfere
with analysis after getting through electrolytic key.
Maybe it should be separate topic but if You mentioned capillary electrophoresis another similar technique is micellar electrokinetic chromatography.
Unfortunately i did not managed to find anything to coat capillary with to make it less brittle than plain glass capillary. |
I don't know what purity the silver wire should be. I guess the about 10% copper and or other metals of sterling silver would change the emf relative
to pure silver but how significantly I don't know.
As to the diameter I would expect the larger the silver area the lower the impedance of the reference electrode would. The impedance of the electrode
will introduce an error due to the current flow to the electronics but modern measuring electronics can easily have a hundred meg ohm input impedance
so I doubt that is a limitation. Probably the limit on how small it can be is your construction limitations.
All reference electrodes potentially contaminate your reaction because they must have the reference electrode/solution in liquid contact with the
external solution. The porous plug is designed to minimise that. The limitation for copper may be that in alkali solutions copper hydroxide form in
the porous plug
I had look up what micellar electrokinetic chromatography was. Interesting using an emulsion as the separation medium.
There is pure silver (claimed to be 99.9%) wire for sale on ebay UK £1.75 (including postage) for 100mm of 0.25mm dia wire see
https://www.ebay.co.uk/itm/999-Pure-Silver-Round-Wire-0-25-m...
I don't think you have to coat a capillary tube in anything to stop it snapping it just needs to be small enough to bend.
I am wg48 but not on my usual pc hence the temp handle.
Thank goodness for Fleming and the fungi.
Old codger' lives matters, wear a mask and help save them.
Be aware of demagoguery, keep your frontal lobes fully engaged.
I don't know who invented mRNA vaccines but they should get a fancy medal and I hope they made a shed load of money from it.
|
|
|