Chemorg42
Hazard to Others
  
Posts: 103
Registered: 12-11-2019
Member Is Offline
Mood: Concentrated
|
|
Ammonium Nitrate in Canada
This has been discussed before, but not directly related to Canada (at least I cannot find anything.)
Does anyone know of any OTC sources or, relatively, safe / inexpensive syntheses for Ammonium Nitrate?
Much obliged
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
Quote: Originally posted by Chemorg42  | This has been discussed before, but not directly related to Canada (at least I cannot find anything.)
Does anyone know of any OTC sources or, relatively, safe / inexpensive syntheses for Ammonium Nitrate?
Much obliged |
Maybe agricultural suppliers might be able to accommodate you?
[Edited on 18-1-2020 by G-Coupled]
|
|
|
B(a)P
International Hazard
    
Posts: 1139
Registered: 29-9-2019
Member Is Offline
Mood: Festive
|
|
I am not in Canada, but some googling shows me your ACE hardware stores stock both ammonium sulfate and calcium nitrate. https://www.acehardware.com/departments/lawn-and-garden/gard...
I can't find ammonium nitrate in Australia so I make mine from two similar products. I make mine by combining dissolved solutions with a slight excess
of calcium nitrate, then once filtered recrystallise it in water to remove impurities and wash with ethanol to remove excess calcium nitrate. I get a
reasonable product from this process, but it is a lengthy one and the initial filtration to get rid of calcium sulphate is painful. Hope this helps.
|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
In my area, shoppers drug mart sells Calcium Ammonium Nitrate cold packs. But other than that, i have found nothing, and searched i have.
I got a small amount of Sodium Nitrate from work a few years back (aquaculture) and i've been using that ever since. I set up an ostwald reactor
recently too, (lifesaver honestly) though I understand the irony of needing some starter nitric acid in order to make the catalyst.
Check garden stores, farm and feeds, if you know any farmers or people who might be able to buy some legitimately (or have some kicking around) start
being nice to them. It is out there, searching around is my only suggestion.
If you don't mind sharing, whereabouts in Canada are you?
"Open your mind son, before someone opens it for you." - Dr. Walter Bishop
|
|
|
Chemorg42
Hazard to Others
  
Posts: 103
Registered: 12-11-2019
Member Is Offline
Mood: Concentrated
|
|
Thanks for all the replies. I am located in the Waterloo region in southern Ontario (for those who asked.)
Also, I just realized that this thread probably should have been posted in apparatus and reagent acquisition, sorry.
|
|
|
Vosoryx
Hazard to Others
  
Posts: 282
Registered: 18-6-2017
Location: British Columbia, Canada
Member Is Offline
Mood: Serial Apple Enjoyer
|
|
Quote: Originally posted by Chemorg42  | Thanks for all the replies. I am located in the Waterloo region in southern Ontario (for those who asked.)
Also, I just realized that this thread probably should have been posted in apparatus and reagent acquisition, sorry. |
Don't worry too much about thread location... There's a lot of overlap with most topics. When in doubt, beginnings, and if there's a huge error the
mods will move it
"Open your mind son, before someone opens it for you." - Dr. Walter Bishop
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1641
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
WallMart equate cal Ammonium nitrate cold packs.
Dissolve in Ammonia water, filter out crap, then dry
I use those to make my Nitric acid (And gives you ammonium sulfate for an ammonia generator)
Or as a bursting charge too.
|
|
|
sclarenonz
Hazard to Self
 
Posts: 74
Registered: 13-12-2015
Location: BRASIL,oiapoque ,amapa
Member Is Offline
Mood: only the mission forget past
|
|
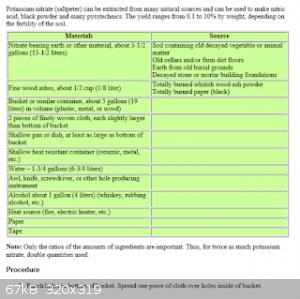 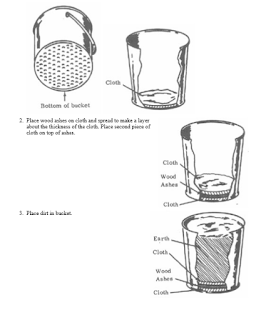 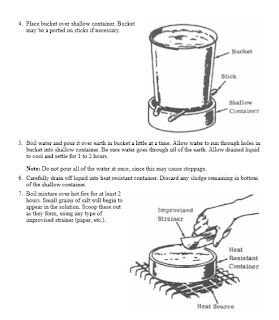 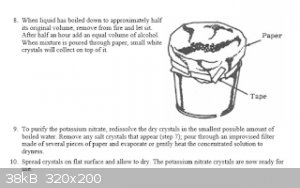
if you find the ureia ,urea is very easy to attract nitrates, in my experiments, I choose a land that is a well-fertilized area, with a lot of
vegetation, I chose the red soil because I know that there has iron and I know that the nitrogenase bacteria use the iron for this, then an
incredible thing happens, after the urea dissolves in the water and I throw it in the land all the water is expelled upwards, when we throw ashes, a
very strong ammonia smell appears, when heat it the nitrous oxide smells appears. thanks
|
|
|
Abromination
Hazard to Others
  
Posts: 432
Registered: 10-7-2018
Location: Alaska
Member Is Offline
Mood: 1,4 tar
|
|
I have a bunch of ammonium nitrate extracted from cold packs. It isn't very clean fresh out of the cold pack, so a few recrystallizations took care
of it. Only problem is it is super wet.

List of materials made by ScienceMadness.org users:
https://docs.google.com/spreadsheets/d/1nmJ8uq-h4IkXPxD5svnT...
--------------------------------
Elements Collected: H, Li, B, C, N, O, Mg, Al, Si, P, S, Fe, Ni, Cu, Zn, Ag, I, Au, Pb, Bi, Am
Last Acquired: B
Next: Na
-------------- |
|
|
Alphachem
Harmless

Posts: 4
Registered: 12-11-2019
Location: Mississauga, ON
Member Is Offline
|
|
Ammonium Nitrate is not something that will be available OTC in Canada at least. It is a highly regulated product that can only be sold to business
providing an end use declaration and business license.
If you are looking for a supplier for any other chemicals please contact us at Alphachem. We are located in Mississauga and have a wide variety of
products in many sizes. We do sell to the general public, however not Ammonium Nitrate
|
|
|
MadHatter
International Hazard
    
Posts: 1352
Registered: 9-7-2004
Location: Maine
Member Is Offline
Mood: Enjoying retirement
|
|
Calcium Ammonium Nitrate(CAN)
B(a)P, I use the same method as you. The CAN is the double salt
decahydrate: 5Ca(NO3)2•NH4NO3•10H2O. The reaction:
5Ca(NO3)2•NH4NO3•10H2O + 5(NH4)2SO4
-->11NH4NO3 + 5CaSO4 + 10H2O
works very well with a maximum stoichiometric yield of 50.56%.
The problem is the thin paraffin coating on the CAN available in the
USA. I'm assuming this is for slow release in soil. I don't know if other
countries coat it. To get rid of this filter ice cold(< 1C) CAN/H2O solution
through paper towels then add the ammonium sulphate.
Then comes the pain in the ass calcium sulphate filtration.
Certainly both precipitates can be filtered at the same time but I don't
like filtering 2 compounds at once.
I use a ratio of 8:5, CAN/Ammonium sulfate, to ensure that the CAN
is used up.
From opening of NCIS New Orleans - It goes a BOOM ! BOOM ! BOOM ! MUHAHAHAHAHAHAHA !
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Find stump remover (containing near pure KNO3) and CuSO4. Mix in proper proportions and freeze out the K2SO4 hydrate leaving aqueous Cu(NO3)2.
Note, for just NH4NO3, just add NH3 (aq) to aqueous cupric nitrate (which also creates a suspension of Cu(OH)2, but with excess ammonia dissolves).
Note, adding excess NH3 may increase the energetic content of the mix (via the adding copper ammine complex also forming a nitrate) and, in any event,
a copper ion impurity in the NH4NO3 makes it less stable.
Otherwise, isolate the copper nitrate hydrate and subject it to a thermal decomposition (caution: corrosive/toxic gas) collecting the exit gases in
water. Aeration can be used to convert any formed HNO2 into HNO3.
Reference: (Chemiday): "The thermal decomposition of copper nitrate to produce copper oxide, nitrogen dioxide and oxygen. This reaction takes place at
a temperature of over 170°C."
2Cu(NO3)2 → 2CuO + 4NO2 + O2
Source: https://chemiday.com/en/reaction/3-1-0-786
Note: the beneficial production of O2 supplements the need for aeration.
One can use also Mg(NO3)2 as per Wikipedia, but it decomposes at 330 C:
"Since magnesium nitrate has a high affinity for water, heating the hexahydrate does not result in the dehydration of the salt, but rather its
decomposition into magnesium oxide, oxygen, and nitrogen oxides:
2 Mg(NO3)2 → 2 MgO + 4 NO2 + O2
The absorption of these nitrogen oxides in water is one possible route to synthesize nitric acid. Although inefficient, this method does not require
the use of any strong acid."
The source of Mg(NO3)2 is even more available being KNO3 + Epsom's Salt (MgSO4 hydrate) followed again by freezing out the K2SO4 hydrate. Note again,
for just NH4NO3, just add NH3 (aq) to aqueous Mg(NO3)2 (which also creates a white suspension of Mg(OH)2, as I have successfully performed and
reported).
The last option is first buying or prepare Aluminum sulfate (from Al + NaHSO4 (aq), and freeze out the Na2SO4) to which is added KNO3 and freezing, as
a path to Aluminum nitrate (which decomposes between 150-200 C liberating again NO2 and O2). Also, the action of ammonia water on Aluminum nitrate
likely forms Al(OH)3 and NH4NO3.
For completeness, why paths to pure HNO3? Because on adding NH3 gas (best in excess to remove all HNO3), the sole product is pure NH4NO3 (without a
copper or other transition metal impurity, which in the case of energetic compounds, can be a problem). To quote a ScienceDirect commentary on this
last point:
"Limitation of impurities is equally essential, since they increase the tendency of the ammonium nitrate to decompose. Impurities which sensitize
decomposition include copper and zinc as well as nitric acid and chlorides. Solid ammonium nitrate is very hygroscopic and tends to cake into a large
mass which is difficult to handle."
[Edited on 24-2-2020 by AJKOER]
|
|
|