DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Bromination not going as expected??
Hello all,
I've been working lately with ferulic acid, a hydroxycinnamic acid derived from plant material, and used as an antioxidant eg in cosmetics. It is
readily available from DIY cosmetic suppliers.
I have performed a couple of conventional reactions, eg esterification and acetylisation on the compound:
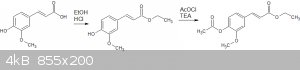
The esterification was following a literature procedure, but I was unable to isolate the ethyl ester separately - it remained an thick oily yellowish
liquid. Rf of 0.61 with 1:3 EtOAc:hexane. I used that product directly, but there was a small amount of ferulic acid remaining. Ferulic acid appears
as a short streak on the plate, to a maximum of about 0.09.
The acetylisation seemed to go smoothly, but I didn't use acetic ahydride as per similar literature reactions, but acetyl chloride and triethylamine.
I ended up with, after two recrystalisations from EtOH, a white powder with a MP of 119-120C, and a single spot on the TLC, Rf of 0.69, same solvents.
Yield to this point was a bit crap, 34%. I think I can improve in places.
Anyway, the main problem I am facing is that I want to now brominate the double bond:
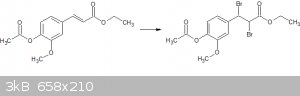
I have tried many different bromination routes, eg H202/HBr, and finally Br2 in chloroform (after doing my first ampoule cracking...). In most cases,
I have recovered my starting material. With the Br2 in chloroform, there are a couple of extra products seen on TLC, but always with a substantial
amount of source product unreacted (same Rf and MP observed). I'm trying to isolate these other products at the moment.
The reaction appears to proceed as expected, with the dark red solution passing through orange to eventually colourless, and I've used an excess of
bromine.
So any ideas? Are there any reaction conditions I should be paying attention to, temperature etc?
And any suggested test I can use to see if any of the products have actually been brominated?
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
You can test if the product is halogenated by fusing a small amount of it with elemental sodium(heat it strongly in a test tube, it will burn, but
thats nothing to worry about). Then dissolving the residue in water and after filtering adding a solution of Silver nitrate. If there is a
halide(except fluorine) present it will form a precipitate of the corresponding silver halide.
Regarding your reaction itself what confuses me is, that the bromine is used up without halogenating the compound. In general these halogen additions
to alkenes are going quite fast and without any problem. Just think about bromine water, that after dumping in a bit of an alkene goes colorless in no
time. But if the compound really isn't halogenated the color of your bromine shouldn't fade. The only exception should be if there is any reducing
compound present converting the bromine to bromide, but the compound doesn't seem to have any reducing groups. Are you sure, that you did your
calculations right and used enough bromine?
|
|
|
BromicAcid
International Hazard
    
Posts: 3253
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Looks like it could be part of the conjugated system coming from the ring which may confer some additional stability. Bromination in general doesn't
give the best yields and bromination of alkenyl esters isn't any better so you're probably aiming for south of 50% to start out with. However, visual
indications are that the bromination is progressing, what percent starter are you recovering? Is it possible that you're just getting really poor
yields? You're making sure to keep light away from your reaction to prevent free radical bromination of your aromatic, correct? Especially
considering your benzene ring appears activated with all that electron density sitting around.
[Edited on 12/30/2019 by BromicAcid]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by DrDevice  |
I have tried many different bromination routes, eg H202/HBr, and finally Br2 in chloroform (after doing my first ampoule cracking...). In most cases,
I have recovered my starting material. With the Br2 in chloroform, there are a couple of extra products seen on TLC, but always with a substantial
amount of source product unreacted (same Rf and MP observed). I'm trying to isolate these other products at the moment.
The reaction appears to proceed as expected, with the dark red solution passing through orange to eventually colourless, and I've used an excess of
bromine.
So any ideas? Are there any reaction conditions I should be paying attention to, temperature etc?
And any suggested test I can use to see if any of the products have actually been brominated? |
Can you be more detailed in describing your protocol? Things like concentrations, solvents, and temperatures would be helpful to know for
troubleshooting.
And what analytical methods can you use? IR, NMR, GC-MS would all be able to tell if brominated product is present.
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Thanks for the suggestions so far.
I don't have any quantatative values to hand for the amount of unreacted material - just trying to separate out the individual components at the
moment.
To prepare the bromine solution, I chilled 59g of chloroform in the freezer, then added the contents of one ampoule of bromine. Weighing afterwards
indicated I had 52.3g of bromine with 59g of chloroform, ie 5.94mmol/g of solution. I'm not confident of my ability to store "neat" bromine
effectively, thus the use of chloroform. Not sure if that is any better, mind you...
For the last reaction, I dissolved 1.6g / 6.1mmol of the ethyl ester in 20ml of chloroform.
1.1g (6.5mmol) of the cold bromine solution was added dropwise to the ester solution at room temperature with magnetic stirring. Addition took a
couple of minutes. No attempt was made to exclude any light - room lighting was fluorescent tubes. Stirring was continued for 2.5 hrs, all of the
colour had gone within 2 hours.
And I've just realised that I need double the amount of bromine I used...D'oh!
I have zero analytical methods available to me 
Once the weather cools down a bit, I'll try the reaction again with light excluded. AND THE CORRECT AMOUNT OF BROMINE!
|
|
|
AvBaeyer
National Hazard
   
Posts: 651
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
First, 1 mole of bromine will brominate 1 mole of olefin. You do not need any excess bromine. The reaction with the double bond should be nearly
instantaneous.
Second, bromination of the double bond will yield two diasteroisomeric dibromides - erythro and threo. Check any good organic chem text to determine
why this is so. The two isomers generally have quite different properties eg melting point, tlc behavior.
I have brominated several cinnamic ester derivatives using methylene chloride as solvent. This have been done in two phase systems. The bromine is
generated in a water solution with the substrate in the CH2Cl2 solution. The bromination proceeds well with good stirring of the two phase system.
Yields of the combined isomers have always been in excess of 90%. This process eliminates the need to obtain and handle elemental bromine in a home
lab setting.
Finally, the reaction of acetyl chloride with triethylamine rapidly generates ketene (well known in beta-lactam chemistry) which in turn can undergo a
variety of reactions, only one of which is likely acetylation of your phenolic oxygen. Thus your poor yield is probably due to the use of
triethylamine as base. Pyridine would be a much better choice as base and acetic anhydride as acetylation agent.
AvB
|
|
|
DrDevice
Hazard to Self
 
Posts: 75
Registered: 19-3-2012
Member Is Offline
Mood: Incompatible with carbon based lifeforms
|
|
Success, I think.
1.35g (5.11mmol) of the ethyl ester of o-acetyl ferulic acid was dissolved in 20ml chloroform. To this was added dropwise, with continuous magnetic
stirring at room temperature, 1.77g of a solution of bromine in chloroform, containing 5.94 mmol/g of bromine.
The addition was performed in as low light as possible, with aluminium foil surrounding the beaker.
After 2 hours, the solution was colourless. TLC showed two spots, one at 0.7, the other at 0.81. I was concerned at this stage because the spot at 0.7
lined up exactly with the source material.
After evaporation of the chloroform, I was left was a clear gel/solid. On addition of EtOH with stirring, a white powder separated. This was filtered
and washed with more EtOH. The powder was allowed to dry and a yield of 0.63g was obtained. MP = 124-125C.
The EtOH was evaporated from the filtrate and left 1.41g of a viscous yellowish liquid.
TLC on the components showed that the "white powder" product had an Rf of 0.78, and the viscous liquid Rf was 0.71, approximately aligning with the
source material. There was an additional faint spot corresponding to the "white powder" material on the viscous liquid trace.
Total mass of both products (as AvBaeyer says, likely the two diasteroisomers) is 2.04g. At a MW of 424.08, this is 4.8mmol, or a yield of 94%.
I had previously tried the two-phase method of generating this compound, with HBr/H2O2, but with ambiguous results. Is it likely that the exclusion of
light played a large part in the success of this reaction? Its the only thing I can think of that was different from previous attempts.
I will repeat with the two-phase approach with light exclusion to see if I get similar results.
Thanks all for the help.
[Edited on 1-1-2020 by DrDevice]
[Edited on 1-1-2020 by DrDevice]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Or you can
do it the easy way.
https://en.wikipedia.org/wiki/Beilstein_test
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Copper wire and a methylated spirit burner (see above).
|
|
|
Tellurium
Hazard to Self
 
Posts: 84
Registered: 12-7-2017
Location: Group 16, Chalcogen City
Member Is Offline
Mood: smelly
|
|
Oh sure, didn't had that in mind right know, thanks. Yes that's a way easier way to test for halogenation. The sodium method is just better if you
wan't to know which halogen you have in your compound, which he obviously does know 
|
|
|