Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Patience and Rubazonic acid
Back in 2016 I was making azo dyes from 4-amino-antipyrine (1-phenyl-2,3-dimethyl-4-amino-pyrazol-5-one) and decided to try making similar dyes from
the closely related 1-phenyl-3-methyl-4-amino-pyrazol-5-one instead. My plan was to nitrosate 1-phenyl-3-methyl-pyrazol-5-one with sodium nitrite and
hydrochloride acid and then reduce it with zinc and acetic acid. The first stage of this scheme worked fine yielding a beautiful orange nitroso
product but the reduction gave just a brick red exceedingly insoluble powder that I assumed was a substituted bis-pyrazoloneazo compound by analogy
with the reduction of nitro or nitrosobenzene. I eventually discovered that the compound can be recrystallised from ethyl acetate to give beautiful
scarlet red blades up to several cm long.
However, this azo compound was amazingly resistant to further reduction to the amino compound. After 3 years of occasionally plugging away at this
compound I have finally identified it almost beyond doubt as a compound know to earlier chemists as rubazonic acid:
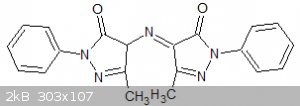
One pyrazolone ring contains one more hydrogen than the other but numerous tautomeric structures are possible. Traditionally it was formed by the
atmospheric oxidation of the aminopyrazolone but it appears that it can also be formed by the partial reduction of the nitroso compound.
The point here is that is is sometimes worth persevering even with an apparent failure. Rubazonic acid is to 1-phenyl-3-methyl-pyrazol-5-one what
purpuric acid is to alloxan.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Interesting work! I wouldn’t have expected that such a compound even existed. Does it only come in red or do salts have different colors?
[Edited on 15-12-2019 by clearly_not_atara]
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
That's really cool - even the structure looks pretty awesome, like some kind of Star Wars experimental fighter. 
How did you finally identify the compound?
[Edited on 15-12-2019 by G-Coupled]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I seem to remember that the nitroso compound was a deep green color.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I haven't yet got round to investigating the salt forming abilities of rubazonic acid but it probably forms anionic salts by deprotonation of the
enolized carbonyl on the left hand pyrazolone ring. It may also form cationic salts with mineral acids. I can't be 100% sure of its identity but in
the older literature there are several references to it and the common thread is its mode of formation which would fit roughly with the method I used
to prepare it (the amino derivative is reportedly very unstable and gives rubazonic acid on atmospheric oxidation). It has a sharp melting point
according to the literature but my MP equipment is not working at present so this test will have to wait. The most characteristic feature is the
inability of this compound to be reduced to an primary amine which would be the case were it a simple azo compound.
My original feeling was that the compound was an azo compound formed by the condensation of the 4-aminopyrazolone with unreduced 4-nitrosopyrazolone:
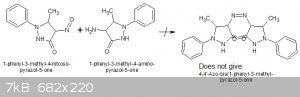
But the properties of the product, apart from its colour, do not fit an azo compound.

Lovely colour: possible rubazonic acid
For proof of its identity I am going to try and synthesize it from equimolar amounts of 1-phenyl-3-methyl-pyrazol-5-one and its 4 nitroso derivative
with the aid of either sodium methoxide or an acid:
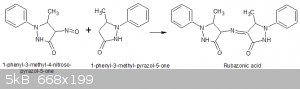
There are examples of such reactions, as in the preparation of nitrobenzaldehydes by the condensation of the corresponding nitrotoluene with
nitrosodimethylaniline in the presence of sodium ethoxide and hydrolysising the resulting imine.
The 4-nitroso derivative of 1-phenyl-3-methyl-pyrazol-5-one is actually orange while the the 4-nitroso derivative of antipyrine
(1-phenyl-2,3-dimethyl-pyrazol-5-one) is deep greenish blue and can be reduced to a simple amine which can be diazotised and coupled to phenols.

Blue-green nitroso-antipyrine (left) and orange 1-phenyl-3-methyl-4-nitrosopyrazol-5-one but the photograph does not do the colours justice.
[Edited on 15-12-2019 by Boffis]
[Edited on 15-12-2019 by Boffis]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Ah I see I mixed up antipyrine and edavarone, thanks for clearing that up. Beautiful colors indeed! Boffis can I ask why you are working on these
compounds?
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Because they are pretty, I would guess. Great colors there. I have made some pyrazoles before and even more similar compounds, but none that had
any real color other than off whites, so I am jealous. Very nice, and patient, prep.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
No not because they are pretty! I was trying to prepare the respective amino compounds because, at least in the case of 4-aminoantipyrine, they form
diazonium salts which couple with phenolic compounds like chromotropic acid, indazole, naphthols, resorcinol etc to form dyes which combine with
metals in solution and produce either precipitates or characteristic colours.
|
|
|