Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Diethylamine and m-Toluic acid from DEET
As promised my write-up on the alkaline hydrolysis of DEET is attached. Any comments, typos, or other errors please let me know.
I have pushed the recovery of the toluic acids further and now have a few more grams of barium m- and o-toluate and finally with the volume of liquor
down to less than 30ml glassy prisms of the barium p-toluate have finally appeared. There obviously isn't a lot of the p-isomer present as it is
sparingly soluble anyway so for it to be the last to crystallise it must be a very minor component (1% or less).
Attachment: Diethylamine and m-toluic acid from DEET rev0 Boffis 2019.pdf (777kB)
This file has been downloaded 1744 times
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Nice presentation. Thank you.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Does m-toluic acid really have a honey odor?
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Not to me, it just irritates the mucous membrain slight.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Very nice write up, much better than in the thread referenced. Also much more practical to be able to perform this in a distillation apparatus at
ambient pressure than a stainless steel pressure vessel!
Did you notice any etching of the glassware, concentrated lye has a tendency to attack glass, especially on heating, not a serious problem?
Also what are your thoughts on doing the hydrolysis under distillation set up, instead of under (air-cooled) reflux or without condensor?
In the small scale prep there are molar amounts mentioned, but written as molar concentration, small mistake, still perfectly understandable.
In the large scale prep these are omitted. for up and down scaling its very convenient to see these, could you write down molar amounts and
equivalents for reagents and volumes (x ml per y g(or mL or mol) of reactant z) for solvents? Preferably also in the work-up. Indicate on what the
volumes are based on once then keep using that as a reference.
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Good write-up, it is similar in style to those early 1900-s reports that the great chemists of those days were publishing in the periodicals of the
Royal Society. (In case a non-native English speaker can say anything meaningful on writing style.)
I found a minor typo in the text (S.C.Wack is the correct name, not S.G.Wack), but it is just nit-picking. (But may serve as an indicator that I
really read what I'm trying to criticize.) 
Is it just me or you really omitted the use of a thermometer (refluxing temp of the reaction mixture). What about the melting points of the toluic
acid isomers in various stages of purification? I well know the misleading nature of absolute melting points alone, but in a purification process the
gradual rising of this parameter may be interesting to note.
All in all, you set a new standard for me what I'll try to adhere to in my coming prepublication articles. (Not the one I'm currently working on,
errhhmm..  , but the ones from the unforeseeable future.) , but the ones from the unforeseeable future.)
Sigmatropic: in my experience I only got serious (visible, touchable) damage to my flasks when I was careless and boiled undissolved, solid NaOH in
them, especially when it was caked and attached to the glass, and the stirrer could not move the lump. Distilling e.g. dimethylamine from strong,
alkaline solution left a spotless flask behind. (Although I did not check its weight before and after the torture.)
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Very cool! Just a minor nitpick: you often write "M" which means "molar", where you mean moles. For example:
| Quote: | | My initial trial with alkali hydrolysis used 10ml of 95% DEET (roughly 0.05 M) in 20ml of 50% aqueous potassium hydroxide (roughly 0.17 M) and 30ml of
ethylene glycol. |
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Hi Sigmatropic, thank you very much for your comments.
As for etching of the glassware by the hydrolytic medium, yes there is quite significant etching of the flask I used for the hydrolysis. Its not
enough to compromise the flask yet but when the flask is dry it is clearly visible. I think the flask would be good for quite a few such runs. I was
more worried about getting alkali in the ground glassy joints as this would probably permanently sieze them after 10 hours, even when greased.
I did the hydrolysis under distillation conditions. The diethylamine slowly distils off as it is produced until you get close to the end when for some
reason it seems to stop distilling but you can overcome this by adding some water and quickly distilling off the last of the amine. As I explained I
used the amount of amine that had distilled off as a means of monitoring the reaction by adding a pH indicator to the collection water and a known
amount of HCl. The still head never became too hot to touch in spite of the glycol mixture appearing to boil, I suspect that this is simply due to the
fact that the diethylamine boils at about 55 C though a little water is clearly carried over with it. The water may be an essential component of the
vapour and this may be why you have to add a little water at the end to distil off the last of the amine even though the flask is by then at perhaps
160 C. Too much water at the beginning inhibits the reaction.
I could certainly work out molar amounts for the reactant but I find (and I think many amateurs) that simple weights are more convenient to work with
even when scaling and experiment. Generally the only time I work exclusively in molar quantities is when I am working on "library" preparations, where
I will replace one or more reactant for each subsequent synthesis. In the initial experiments I did work with molar amounts because I wanted to be
sure that I had sufficient concentration/excess of say alkali while changing the type of alkali. In the later experiments I based the quantities on
what I had learned from the earlier experiments. I will look at adding these values for both the hydrolysis and the amine work up and for the toluic
acid work up where feasibles. In the latter case I was very much doing it by eye, thats why I didn't include the final work up and recovery of the
o-toluic acid. Here you evaporate the barium salt solution a little and see what shape crystals you get while warm, filter very quickly and see if you
get the same crystals when cold (you often don't) and then repeat the process over and over again. All the tiny crops of crystals of one shape are
then mixed together and recrystallised. After the first crop you are then back to the incremental evaporation. I'm not doing this to recover the acids
only to estimate the amounts and nature of the impurities! Its difficult to quantify this procedure and I don't think most SM members are patient (or
masochistic) enough!
@Metacelsus, That's a good point and I'll fix it when I issue rev1. Should I spell out "moles" or can I use an abbreviation like "mol."
@Pumukli, I missed your reply initially, sorry. Thanks for the response. The reason there isn't a lot of use of thermometers was because I didn't want
to risk my precious thermometers in hot caustic liquids! I am not sure that in the case of such brutal hydrolytic conditions vapour temperatures are
very meaningful.
You are right about the final distillation of the amine, it didn't etch the flask I used at all but then again I used only a very small excess of
alkali and the whole distillation takes perhaps 15mins at a maximum of 60C. The flask I used for the two final production runs was exposed to much
stronger KOH, much higher temperatures and 20 hour of boiling. Considering these conditions I didn't think the etching was so bad, the flask is still
servicable.
[Edited on 7-12-2019 by Boffis]
[Edited on 7-12-2019 by Boffis]
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by Boffis  | @Metacelsus, That's a good point and I'll fix it when I issue rev1. Should I spell out "moles" or can I use an abbreviation like "mol."
|
"mol" would be fine.
|
|
|
stoichiometric_steve
National Hazard
   
Posts: 827
Registered: 14-12-2005
Member Is Offline
Mood: satyric
|
|
I am in awe of the sheer volume of words and pictures. Who would have thought you can squeeze 11 pages out of a simple basic hydrolysis?
|
|
|
Mush
National Hazard
   
Posts: 633
Registered: 27-12-2008
Member Is Offline
Mood: No Mood
|
|
Brilliant! Thanks again for the hard work.
|
|
|
njl
National Hazard
   
Posts: 609
Registered: 26-11-2019
Location: under the sycamore tree
Member Is Offline
Mood: ambivalent
|
|
What role does the EG play here? Is it just a solvent?
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Yes, ethylene glycol is a good high temperature, protic solvent that is often a better solvent for organic substances than water and it also dissolves
NaOH and KOH.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
Excellent write-up!
As elucidated in my recent thread, I am going to try this soon.
I have a quick question, but it isn't too important:
Is there any particular reasoning behind the quantity of alkali you used?
51g of KOH for 45mL of DEET seems to be a 4x excess, is this necessary?
Furthermore, if less alkali could be used, it would probably not be necessary to use any water to dissolve it, it would probably dissolve fully in the
glycol.
I will be using propylene glycol and NaOH so I'm not sure how well it'll work, we shall see!
|
|
|
ErgoloidMesylate
Banned
 
Posts: 89
Registered: 8-8-2022
Location: Norad
Member Is Offline
Mood: Freedom of thought is priceless - You can't afford it
|
|
Quote: Originally posted by SplendidAcylation  | Excellent write-up!
As elucidated in my recent thread, I am going to try this soon.
I have a quick question, but it isn't too important:
Is there any particular reasoning behind the quantity of alkali you used?
51g of KOH for 45mL of DEET seems to be a 4x excess, is this necessary?
Furthermore, if less alkali could be used, it would probably not be necessary to use any water to dissolve it, it would probably dissolve fully in the
glycol.
I will be using propylene glycol and NaOH so I'm not sure how well it'll work, we shall see! |
I'm sorry, but it seems or sounds like you said you wanted to do a hydrolysis without water.
::Scratches head::
Cannula transfer and cannula filtering is used for lysergic synth Compressed air and remove co2
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Ever had a look at that mechanism? It doesn't use water.
|
|
|
SplendidAcylation
Hazard to Others
  
Posts: 203
Registered: 28-10-2018
Location: Starving in some deep mystery
Member Is Offline
Mood: No one I think is in my tree.
|
|
@EM I have since carried out the reaction, and it was quite successful, however it did quite a bit of damage to the flask.
I have a write-up on the way in case anyone is interested, should be ready soon.
Yeah it isn't hydrolysis, but then again, many reactions are called "hydrolysis" even though the main reaction taking place doesn't involve water.
For instance the "hydrolysis" of an ester, or an acyl halide, with dilute NaOH.
The hydroxide ion is so much more nucleophilic than water, so the water will hardly participate at all, but it is convenient to call it hydrolysis.
The DEET reaction, however , can be more accurately called alkaline decomposition 
@tsjerk I think the reaction mechanism involves nucleophilic attack on the amide carbonyl, resulting in an amide anion as a leaving group.
The amide anion is a stronger nucleophile than hydroxide, so the reaction requires forcing conditions such as an excess of alkali, or distillation to
remove the amine...
I hope I'm right, I spent long enough working on this reaction!
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Hydrolysis can occur without water, sodium hydroxide dissolved in some other solvent will do the trick just fine. Hydroxide is just deprotonated
water.
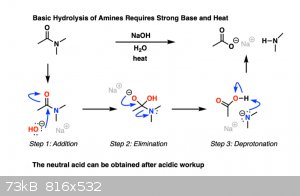
https://www.masterorganicchemistry.com/2019/10/07/amide-hydr...
|
|
|
ErgoloidMesylate
Banned
 
Posts: 89
Registered: 8-8-2022
Location: Norad
Member Is Offline
Mood: Freedom of thought is priceless - You can't afford it
|
|
Quote: Originally posted by Tsjerk  | Hydrolysis can occur without water, sodium hydroxide dissolved in some other solvent will do the trick just fine. Hydroxide is just deprotonated
water.
|
The hydroxyl ion your saying would pass muster.
Water being present certainly wouldn't hurt.
Cannula transfer and cannula filtering is used for lysergic synth Compressed air and remove co2
|
|
|