chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Methoxy-EthylTryptamine
I’m considering trying to synthesize some new tryptamines from substances such as melatonin and tryptophan.
I’ve decided, just as an introduction to the project, that I want to make methoxy ethyl tryptamine, a substituted tryptamine, where in the 5
position there is a methoxy group, and one of the hydrogens on the primary amine is replaced by an ethyl group. Here’s a method I think might just
work:
I’ll take methoxy-AcetylTryptamine(melatonin) and react it with hydrazine and a base to reduce the acetyl group to an an Alkane, which will be my
ethyl group. Basically just your standard Wolff-Kishner reduction.
I’m thinking this might work, but I thought I’d get your guys’s Opinions on it first. Let me know if you think this is a doable synthesis, and
if it’s not, let me know why. I don’t have a history for being good at this kind of stuff, but I’d thought I’d maybe give it another go.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Wolff-Kishner reductions are for ketones and aldehydes, not amides. Your plan is doomed to fail.
And frankly, since you didn't know that, you're definitely not experienced enough to use hydrazine.
LiAlH4 might work, but again, I don't think you're experienced enough to safely use it.
[Edited on 2019-11-21 by Metacelsus]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
But u can use Sodium bis(2-methoxyethoxy)aluminium hydride.its a non air sensitive form of LAH in toluene.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I'd think ethylation of the acetamide followed by hydrolysis is easier than aluminum hydride reductions.
I also seem to remember a thread on here that described that basic hydrolysis isn't as straightforward as one might think. Something about rigorous
exclusion of oxygen IIRC. I'm sure somebody in that thread thought about or tried acid hydrolysis
|
|
|
Texium
|
Thread Moved
21-11-2019 at 10:33 |
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Do u mean attach an ethyl to the acetyl hydroxy group and then using what?
Were you thinking Na in alcohol? would that work on a indole ?
|
|
|
chemist1243
Hazard to Others
  
Posts: 170
Registered: 7-8-2019
Member Is Offline
|
|
Quote: Originally posted by draculic acid69  | Do u mean attach an ethyl to the acetyl hydroxy group and then using what?
Were you thinking Na in alcohol? would that work on a indole ? |
no, my end product will be an acetyl group without the oxygen, which is just an ethyl group.
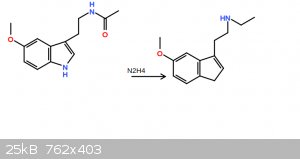
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Sigmatropic's suggestion is an interesting one. It seems that you can alkylate an amide NH if you use an alkylhalide and a strong base. Question is
whether the rest of the molecule would survive this treatment. 
Metacelsus already told that you can safely forget hydrazine reduction in this case. It won't work on an amide.
Draculic acid69's question is unclear for me: what sort of "acetyl hydroxy group" are you talking about?
Now please continue the discussion from here. 
|
|
|
karlos³
International Hazard
    
Posts: 1520
Registered: 10-1-2011
Location: yes!
Member Is Offline
Mood: oxazolidinic 8)
|
|
I second Sigmatropics suggestion.
You have a perfectly clean protected amide to monoalkylate, so why not use this?
I think Na/EtOH will reduce the pyrrole double bond to indoline.
Using hydrazine will, as already pointed out, not reduce any carbonyl group just because it is one, it will only work on ketones not amides.
[Edited on 22-11-2019 by karlos³]
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Maybe Na/EtOH reacted separately /NaOEt in ethanol/ would do the trick.
Edit: On second thought: no  The NH in the indole ring is more acidic than the
amide NH (I feel, not knowing the actual pKa-s !), so it would be alkylated first. The NH in the indole ring is more acidic than the
amide NH (I feel, not knowing the actual pKa-s !), so it would be alkylated first.
[Edited on 23-11-2019 by Pumukli]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Pumukli, your right I totally missed the fact you can alkylate Indoles under the same conditions. And I share your feeling for pka, but this is
organic chemistry so I had to look it up.
Assuming analogy to N-methyl acetamide, the pka of the amide 25.9. https://www.chem.wisc.edu/areas/reich/pkatable/
Indole NH is at 21 so it is more acidic.
Perhaps the dianion strategy can be used but I've never had that succeed, although I never really tried.
Edit: dianion strategy doesn't work and gives alkylation of the indole according to this source.
[Edited on 23-11-2019 by Sigmatropic]
Attachment: v78-265.pdf (374kB)
This file has been downloaded 404 times
|
|
|