numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
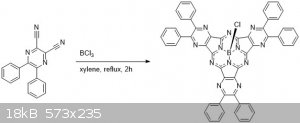
Synthesis of a Highly Fluorescent Boron subporphyrazine
This was originally a fun weekend project for my girlfriend and I, but the results were so pretty I decided to post the pictures here.
Boron phthalocyanines are pretty common dyes nowadays, and I came across a recent paper1 that outlined an easy 1-day synthesis of a rather
novel derivative. The synthesis is straightforward, a simple reaction between a substituted pyrazine and BCl3. The
diphenylpyrazinedinitrile can be easily made by condensing benzil and diaminomaleonitrile.
All glassware was pre-dried in an oven and m-xylene was stored over sieves for a day prior to use. This reaction isn't super air-sensitive, so
rigorous schlenk technique was not used.
To a 20 mL vial with stir bar and septum was added 5,6-diphenylpyrazine-2,3-dicarbonitrile (600 mg, 1 eq). The vial was evacuated and refilled with N2
three times and m-xylene (2.5 mL) was added. Boron trichloride (2.5 ml of 1M solution in DCM, 1.18 eq) was slowly added via a syringe and the vial
heated to 120C with stirring. A needle was placed into the septum and DCM was allowed to boil away under N2 pressure. When all the DCM boiled away,
the purging needle is removed and the reaction is heated for 3 h further under N2. TLC (DCM) after an hour shows formation of product. There are
several other species also visible under UV, so you can't get away with just a plug.
After 3 h the reaction is allowed to cool, giving a black tarry mess. We subjected this goop to column chromatography on silica, but next time it'd be
smart to run it through a plug first. There's so much black stuff it's hard to see when the product comes off. We ended up having to redo the column.
The pic below is the second (much cleaner) column, along with some beautiful fractions.
I decided to keep all the fractions containing the compound since I wanted to recrystallize it anyways.
 
The fractions were combined and the solvents removed under reduced pressure.

This gave a hot pink powder, probably ~95% pure. (44 mg, 7% theoretical vs 6% lit. yield... just not a high yielding reaction)
We really wanted crystals so we crystallized the powder by layering DCM/EtOH. When we came back, the entire vial had turned dark blue, however
filtration afforded nice pink needles. I also concentrated the blue solution and kept that, no clue what it is, maybe the ethanol adduct? It seems to
revert back to the desired compound when left dissolved in DCM, but I did no real characterization on it.
 
I gave the crystals to my gf who was able to get some nice microscope pictures. They are definitely tiny, but needles nonetheless.
![pink2[12215].jpg - 213kB](https://www.sciencemadness.org/whisper/files.php?pid=624976&aid=77777)
Under ambient and 365 nm light:
 
Ref:
1. Dyes and Pigments 2019, 162, 888.
[Edited on 10-26-2019 by numos]
|
|
|
j_sum1
Administrator
       
Posts: 6338
Registered: 4-10-2014
Location: At home
Member Is Online
Mood: Most of the ducks are in a row
|
|
That pink (photo 6) is really pretty. The blue next to it is lovely too.
Great synth. Thanks for sharing this.
|
|
|
Metacelsus
International Hazard
    
Posts: 2542
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Very cool! And I'm glad your GF likes science 
Is the blue solution fluorescent?
|
|
|
G-Coupled
Hazard to Others
  
Posts: 287
Registered: 9-3-2017
Member Is Offline
Mood: Slightly triturated
|
|
That's some groovy chemistry. Nicely done! 
|
|
|
Boffis
International Hazard
    
Posts: 1881
Registered: 1-5-2011
Member Is Online
Mood: No Mood
|
|
Very nice preparation, pitty I don't have any BCl3 or I would have a go too. I have benzil and diaminomaleonitrile though and I wonder if boron could
be subsituted by something else. The dicyano compound has the same motif as o-dicyanobenzene which can give phthalocyanine type compounds.
|
|
|
numos
Hazard to Others
  
Posts: 269
Registered: 22-2-2014
Location: Pasadena
Member Is Offline
Mood: No Mood
|
|
Yes that's correct, I actually am working on getting the o-dicyanobenzene to make the corresponding analog. The original reason I made that pyrazine
was to make some rare-earth coordination complexes. In these cases you actually get a larger phthalocyanine with 4-fold symmetry. I never got around
to making those, but if you have some rare-earth salts laying around, might give that a try! 
Metacelsus, I got lucky, she's a chemist herself! As far as I can tell, the blue solution/compound has no fluorescence.
|
|
|
Boffis
International Hazard
    
Posts: 1881
Registered: 1-5-2011
Member Is Online
Mood: No Mood
|
|
Hi Nimos, Keep us posted on how you get on I have lots of o-cyanobenzene too!
|
|
|