| Pages:
1
2 |
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Processing lead from batteries - finding a method that works
I've been having problems with getting any substantial yields from processing lead acid batteries from car batteries, marine batteries and small SLA's
(UPS backups and such). I find that on average it's possible to get about 15-25% of the metal weight in the battery into a pure lead metal from
melting (and it requires unusually high and long levels of heating for lead). This seems to be a common issue people have found and there are many
video's with people finding how little lead is recovered from this method - so I'd like to see if anyone else has gone through the process and
recovered the full weight of lead from a battery (minus O2 for PbO2 plates).
The plates are supposed to be a plate made of Pb and one of PbO2 (with a Pb lattice underneath that gets converted partially to PbO2). I've read and
been told that the lead used is a sponge lead b/c it has more surface area. I've tried heating the "pure" lead plates maybe 6-8 different ways from
electric, propane, kilns, forges (gas/coal/charcoal) and I usually use a caste iron container, stainless, steel, porcelain, (I've probably tried many
with different heat sources).
I'd have to guess that less than 50% of the weight from the pure lead plates can be recovered by heating, and it takes A LOT more heat than melting
other things like sinkers, wheel weights, bullets, etc. The plates often have to get orange hot before any beads of the lattice start melting out and
I have to guess that most of the melted lead is from the lattice with very little from the sponge. After heating I'm left with a strange almost
powdery lead that has remarkably little PbO for how long and how strongly it was heated. I've had the lead orange/yellow hot, with a fair size pool in
the bottom and even submerging the unmelted lead in this pool doesn't melt it (and it was the same color/temp).
I've dissolved the "pure" lead in vinegar + H2O2 and it completely dissolves and gives beautiful, pure looking lead acetate crystals. I've been told
there may be some antimony in the lead (some say there is, some say it is very pure lead, so IDK) but that also makes a white acetate powder but from
the crystals formed, it looks to be pure lead.
The PbO2 is very difficult (and dirty) to work with and I've tried many of the same things as above as far as heating, with hopes of turning it into
PbO, and I've had difficulty completing this as well. I've tried grinding into a powder and heating it as plates and I did heat until strong orange
color, so it should have been MUCH hotter than needed to reach decomp temps to PbO, and I maintained the heat for about an hour (3 small plates 1" x
2" - so pretty small) which I would think is plenty of time to decompose to PbO, especially since it supposedly only needs about 600F to decompose.
When it gets red-orange hot the powder moves almost like a liquid but I can tell it isn't melted and is still a powder.
So I've tried heating the PbO2 with carbon - coal, charcoal (powdered) and some other charred/carbonized materials (hard nut shells and more). I've
heated it so hot the coal has turned to coke but the carbon doesn't burn up. I've also tried blowing some air onto the red/orange hot carbon/PbO/PbO2
mix (most was PbO at this point). I used a stainless steel tube and tried heating the air before it reaches the lead (heating inside the tube) and
I've tried blowing through an open flame (the one heating the container w/ flames coming up the sides) and also pushing the tube into the container to
blow air into the bottom, hottest part. The only reaction I found was getting some slight green flames around the edges (from lead or carbon?) -
these flames also started on initial heating of the C+PbO2 for about 1-2 mins.
The most disappointing thing about all of this is the amount of useable lead metal easily recovered. I wouldn't be surprised if a person with little
to no experience might recover 10-15% of the weight of the plates/connectors and an experienced person maybe 20-30%. For lead batteries being so
common and good source of lead, there has to be a way to get higher yields w/o dissolving in acids and then going through reducing steps (which often
leads back to PbO). I'd really like to get this problem solved b/c I know I'm not the only one who has a problem with this and would love to be able
to create a process where this is doable by a determined person - it doesn't have to be "kitchen chemistry" level
This is the Pb plates and would only melt while adding heat from the top with a blowtorch. There were solid chunks that just wouldn't seem to break
up or melt. I had to use a screwdriver to cut it and break it up.
    
The below is PbO2 that was mixed with powdered coal. The color of heat was orange though it looks red in the picture. The PbO2 did mostly convert to
PbO but I didn't see any reduction to Pb after well over an hour at orange heat & trying to blow air on/into it.
  
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
I have 2 old lead batteries and plan to recover lead. I thought to use HNO3 to dissolve all metallic leads as well as it's compounds and then separate
lead from impurities by H2SO4. But thank you for publishing another route. I will be able to compare.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
i've done this twice, and i'm tweaking the technique to make the process easier and faster.
just heating the PbO2 is not enough, it will turn to PbO, and then it will melt (probably what you saw was the liquid PbO).
i also thought of adding charcoal and heating them together, but onestly i didn't try, i directly tried the approach i'm using now.
what i'm doing is the same thing that was done in the past to make iron.
the first model was a "cupula furnace" out of a steel can, and two manual air pumps to pump air in the furnace from the bottom. i would light a small
fire inside the can, and then i eould add charcoal, and lead oxides in layers. the charcoal would be consumed reducing the oxide to metal that would
flow to the bottom of the furnace (where i would tap it off from a small hole in the furnace), this first build was not great, the air was
insufficient and i had to manually pump it for 4 hours befor i eould just give up.
the second model was 4 or 5 soup cans piled and secured one to another to make a long steel tube closed at the bottom. the air was now supplied by an
air mattress pump (those used while camping), this version was a failure as the small diameter of the cans made to much air resistance and the heat
was localized too much.
what i'm planning now is to use bigger cans (coffee cans) and finer charcoal, this should work.
talking about yield, the first battery i did i first melted out the lead metal, i got a muffin mold worth of lead, then i processed rhe oxides in the
cupola furnace and i got another 2 muffins worth of lead but i only used half or less of the total oxide i had (i got exhausted by pumping air by
hand).
this is the method used industrially so it should work, and processing 10kg of lead oxides (from 1 big battery or a few smaller ones) can not be done
in beakers or at lab scale.
@teodor to process 2 batteries you are going to need lots of nitric acid, it is going to get expensive.
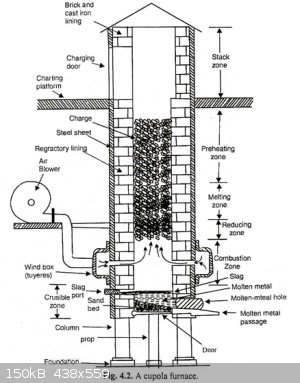   this is the block that came out of the failed run after cooing. you can see the glassy PbO and the little beads of lead on the embedded pieces of
charcoal this is the block that came out of the failed run after cooing. you can see the glassy PbO and the little beads of lead on the embedded pieces of
charcoal
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
What kind of containment are you using? I'd be very worried about lead contamination spreading.
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by teodor  | | I have 2 old lead batteries and plan to recover lead. I thought to use HNO3 to dissolve all metallic leads as well as it's compounds and then separate
lead from impurities by H2SO4. But thank you for publishing another route. I will be able to compare. |
Have you processed any before? It is messy, just beware. I would suggest seperating all the plates and peel off as much paper as you can from
between the layers. Then when you have the lead plates (negative plate) and the PbO2 plates (positive plates). Stack the negative plates, put them in
plastic bags (I used like 8 plastic grocery bags) and wrap them around to get as many layers as possible. Then smash the flat side of the plates -
not as hard as you can but hard enough to break up the sponge. You will be left with lead powder and deformed lattice/matrix.
This lead lattice can be melted easily and recover the lead - as well as the solid top jumper that connects the plates inside the cell and between
cells - that is all good pure lead. Just heat this as normal and put it aside.
The lead powder can then be dissolved easily in acid. The problem is if there is a lot of paper left on the plates before you smash it - I forgot
about that. I actually soaked in water (with a little NaHCO3) and then used a scrub brush & brass wire brush on each side over a bucket to catch
all the lead water (deal with that later - it's not hard, but DON'T put down the drain).
I used vinegar and H2O2 and it makes a grey powder and I think this will dissolve the paper as well - so will HNO3, so this should be avoided. This
could probably be avoided by heating strongly before dissolving and maybe some will even melt and get more pure lead. If you don't scrub well, I'd
suggest this - unless you can get a carbon reduction process to work.
The problem is after this you have the salt, which I think decomps to PbO, which is what you would get from strong heating anyway, so IDK the point of
wasting the acid unless you wanted to make a stronger acid by decomposing the salt.
To deal with the positive plates, I did the same thing, wrapping them in plastic bags. This will SHRED the bags (especially the ones close to the
plates - for both + & - plates) so I'd do this over a ground cover of some kind, the PbO2 gets dustier than the Pb plates. You can also do all
this while the plates are wet/damp and that helps the dust, but wet PbO2 is MESSY and can stain. It should't be too necessary to scrub the PbO2
plates if you are going to heat them to get PbO as it will just burn off.
If you scrub the negative plates & have dirty water left over, allow it to settle (couple days) and pour/siphon off the top 50-70% and discard
then boil down the rest until you have a sludge you can scrape out - which you will then heat/cook to PbO - burning off the carbon. Otherwise you will
have cellulose dissolving in an acid - so IDK what other option there is for this.
There is a way to decompose the PbO by heat but it looks like it needs 2200F which can be difficult to reach w/o special equipment and I'll cover that
in another reply.
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Me? Depends on what and where you are talking about. When dealing with the plates, mine are always wet when handling. All heating is done outside.
When I break any of this up (plates, PbO/PbO2), it's done inside a number of plastic bags, inside 2 layers of large plastic bags that the hammer
& bagged plates are in. Eveything gets treated, put in waste disposal container (special disposal). Gloves when handling the plates (disposable
gloves)
When working with the pure lead? Nothing.
[Edited on 9-25-2019 by RogueRose]
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
lead is dead. just give the battery to a recycling place or the new sealed one to it's manufactures.they give you a discount on a new battery or can
get money.use the money to buy lead from a shooting range. they are much safe to handle and low waste produced.lead from battery are an alloy best for
making them.watch Cody's lab https://www.youtube.com/watch?v=Lkw5UotuRbA
even little of lead ingestion can make you basically dumb, specially you are a kid or a teen.
don't act like you already got lead poisoning and be safe.
melting lead sulfate from a battery releases sulfur oxides.
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Ubya  | i've done this twice, and i'm tweaking the technique to make the process easier and faster.
just heating the PbO2 is not enough, it will turn to PbO, and then it will melt (probably what you saw was the liquid PbO).
i also thought of adding charcoal and heating them together, but onestly i didn't try, i directly tried the approach i'm using now.
what i'm doing is the same thing that was done in the past to make iron.
the first model was a "cupula furnace" out of a steel can, and two manual air pumps to pump air in the furnace from the bottom. i would light a small
fire inside the can, and then i eould add charcoal, and lead oxides in layers. the charcoal would be consumed reducing the oxide to metal that would
flow to the bottom of the furnace (where i would tap it off from a small hole in the furnace), this first build was not great, the air was
insufficient and i had to manually pump it for 4 hours befor i eould just give up.
the second model was 4 or 5 soup cans piled and secured one to another to make a long steel tube closed at the bottom. the air was now supplied by an
air mattress pump (those used while camping), this version was a failure as the small diameter of the cans made to much air resistance and the heat
was localized too much.
what i'm planning now is to use bigger cans (coffee cans) and finer charcoal, this should work.
talking about yield, the first battery i did i first melted out the lead metal, i got a muffin mold worth of lead, then i processed rhe oxides in the
cupola furnace and i got another 2 muffins worth of lead but i only used half or less of the total oxide i had (i got exhausted by pumping air by
hand).
this is the method used industrially so it should work, and processing 10kg of lead oxides (from 1 big battery or a few smaller ones) can not be done
in beakers or at lab scale.
@teodor to process 2 batteries you are going to need lots of nitric acid, it is going to get expensive.
this is the block that came out of the failed run after cooing. you can see the glassy PbO and the little beads of lead on the embedded pieces of
charcoal |
Well I was told that PbO + charcoal would work and it didn't but I've seen video's of people decomposing PbO using a bunsen burner, burnt wooden block
with a cavity in it to hold the Pbo powder, then they would use a blow pipe (glass) to blow air onto the PbO and it turned to lead! It was so simple!
Supposedly copper II oxide - CuO has a MP of 2420F and it can be reduced by H2, CO or C. I have a suspicion that this can be done with lead as well
but I'm really curious if that is true, b/c if that is the case then making water-gas might be the best option to reduce CuO or PbO b/c water-gas is
steam passed over hot coal/coke (IDK if it HAS to be coke) or maybe even charcoal - which produces H2 + CO. so for every molecule of water you should
be able to reduce 2 molecules of metal oxide!
I just found this page that explains H2 reduction & use of water-gas for complete reduction!
http://lead.stcitaly.com/lead-battery-recycling-process/ultr...
I was recently studying refractory material and came across some kind of oven, kiln or forge that was called a reverbatroy oven I think. It's pretty
neat and uses the same principles as sound bouncing off a ceiling to get more heat directed at one spot. Think of two 9"x13" cake pans sitting next to
each other and a ceiling that peaks in the middle between the pans (like an "A" frame roof/ceiling). The flames would burn at the top of the pan and
the flow of the flame/gases would be drawn over the pan next to it and then up a chimney. The roof/ceiling would be angled as such that the radiant
heat would be reflected back down on the non-burning pan, which holds the metal - so it gets the reflected/refracted (IDK which is correct) heat as
well as the direct heat of the flames passing over it. I looked into the materials that could be used and they aren't really that expensive. I think
the three main ingredients were ZrO2, Al2O3, MgO and maybe some binder like sodium silicate (or something that doesn't melt at such a low temp) - it
may have also had calcium silicate in addition or in place of the MgO, I'm not certain. I think the ZrO2 is the most important as it does an
excellent job reflecting/refracting heat - look at pottery supply shops for Zircomax/zirconmax or something similar. Sheffields pottery supply (in MA)
is a great place to look (I know you are in Italy but they have a good list of products to get an idea of prices & what is available.
There are also A LOT of aluminum/silicon based "gem stone" powders that are incredibly refractive and are priced really well. I'll have to find a
list if you are interested in building something to do this, having a non-absorptive refractory will help IMMENSELY with how hot you can get and the
amount of fuel used. Even using simple steel can as an insulator funneling a flame instead of heating hanging over an open air flame, decreased
heating time by at least 50% and increased temp by about 200F.
I'd really like to work on a water-gas generator and figure out the best way to do this, b/c that seems like it might be the most efficient way.
Also, do you know if HCN would reduce metals? There is something that produces large amounts of CO and some HCN and I have A LOT of it, but I need to
know if it would make lead cyanide and how I could neutralize any HCN if it doesn't react. I'm thinking a reaction with a base like bubbling through a
solution of Na2CO3.
Lead reduction videos
https://www.youtube.com/watch?v=Eh8Ll4_xahc
https://www.youtube.com/watch?v=UmL968tIj9U
Copper reduction - not great video though
https://www.youtube.com/watch?v=uxjp4XbsOfU
Copper reduction using H2
https://www.youtube.com/watch?v=0MlMewgsgSk
Kinetics of reduction of lead oxide in liquid slag by carbon in iron
https://link.springer.com/article/10.1007%2FBF02655074
Attachment: Kinetics of reduction of lead oxide in liquid slag by carbon in iron.pdf (824kB)
This file has been downloaded 351 times
Some interesting info from Quora:
https://www.quora.com/What-happens-when-lead-oxide-is-heated...
The Old Metallurgical Engineer says:
Theoretically, PbO + C = Pb + CO(g) or 2PbO + C = 2Pb + CO2(g)
BUT (big but):
Solid-solid reactions are very, very slow (think glacial speed). So, the reduction of PbO with carbon is almost surely:
FIRST:
C + 0.5O2(g) = CO(g) T = 400C
Change in Free Energy: ΔG(400C) = -171.1kJ (negative, so the reaction runs)
Change in Enthalpy: ΔH(400C) = -110.4kJ (negative, so the reaction is exothermic)
THEN:
PbO + CO(g) = Pb + CP2(g)
Change in Free Energy: ΔG(400C) = -56.4kJ (negative, so the reaction runs)
Change in Enthalpy: ΔH(400C) = -10.2kJ (negative, so the reaction is exothermic)
And this reduction of an oxide by CO is exactly what happens to iron ore (iron oxide) Fe2O3 in a blast furnace!
I found a new (2011) Patent Process:
Recovery of high purity lead oxide from lead acid battery paste
https://patents.justia.com/patent/8323595
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Quote: Originally posted by RogueRose  | Quote: Originally posted by teodor  | | I have 2 old lead batteries and plan to recover lead. I thought to use HNO3 to dissolve all metallic leads as well as it's compounds and then separate
lead from impurities by H2SO4. But thank you for publishing another route. I will be able to compare. |
Have you processed any before? It is messy, just beware. |
We melt a lead metal from batteries plates when I was a child, but it was 35 years ago or so. I don't remember whether it was messy, but probably it
was another construction of batteries.
It looks like "Aqua-plomb" from a hardware store 54 EUR per 3.4 kg of lead metal is a better option if I have no plans to dedicate few days of
vacation to sort/recycle battery chemicals but just want to make several lead salts and make experiments with them. Thank you for the instruction, may
be one day, having more experience with lead chemistry I will try to do it from batteries also .
UPD: possible I will still try to disassemble the batteries to see myself what is inside. Well, I can spend 54 EUR for other chemicals 
[Edited on 25-9-2019 by teodor]
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by rockyit98  | lead is dead. just give the battery to a recycling place or the new sealed one to it's manufactures.they give you a discount on a new battery or can
get money.use the money to buy lead from a shooting range. they are much safe to handle and low waste produced.lead from battery are an alloy best for
making them.watch Cody's lab https://www.youtube.com/watch?v=Lkw5UotuRbA
even little of lead ingestion can make you basically dumb, specially you are a kid or a teen.
don't act like you already got lead poisoning and be safe.
melting lead sulfate from a battery releases sulfur oxides. |
Thanks.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
You probably already know this but lead ingots on ebay are like 50-60 cents per pound, shipping included.
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
That works out if You are in US, I had a terrible experience getting lead metal in EU, 10$/100 g on ebay, My hand did not raise high enough to pay
that price, so I ended up extracting lead from gel Pb battery. I don't remember which one was it, but I think you have to have it 100% charged for
most recovery. Well I did it in my flat kitchen in a pot and gas burner. quite easy once you get trough plastic of battery.
|
|
|
Steam
Hazard to Others
  
Posts: 238
Registered: 25-3-2014
Location: Minnesota
Member Is Offline
Mood: Triple Point
|
|
Perhaps this video from Cody's Lab might help! https://www.youtube.com/watch?v=Lkw5UotuRbA
DISCLAIMER: The information in this post is provided for general informational purposes only and may not reflect the current law in your jurisdiction.
No information contained in this post should be construed as legal advice from the individual author, nor is it intended to be a substitute for legal
counsel on any subject matter. No reader of this post should act or refrain from acting on the basis of any information included in, or accessible
through, this post without seeking the appropriate legal or other professional advice on the particular facts and circumstances at issue from a lawyer
licensed in the recipient’s state, country or other appropriate licensing jurisdiction.
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Well, we did it much simpler and with less equipment than in the video. We put lead into a tincan (actually ironcan) and put the tincan into a usual
bonfire with help of some instrument like wrench, on the charcoal. For molding we just used a hole in the ground. It gave not shining surface but
pretty smooth. The rest of the broken battery we just kept in a cellar, under a workbench. Also we had 8mm movie camera but never made a video of this
process.
The original topic deals with PbO2, not with plain lead which is processed at least from 7000 BCE without troubles
.
[Edited on 26-9-2019 by teodor]
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Well, I'd appreciate if people stuck to the title, if you want to post where to find lead bullets, or buy it, there is a whole forum to post those in,
please feel free. But for the people who actually want to understand how it is done, then please feel free to discuss the process here. I'm not sure
everyone has access to a "limestone quarry filled with lead bullets" or a range that will sell lead (it's "hazardous" remember..). So there are
people who want to know how to do it from batteries, and considering 2-3 batteries equals what took cody all day panning for in a shooting range,
people might want to go that avenue. So, please no more derailing the topic. If you want to add to the original premise of the post, please go ahead.
Thanks
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
in the near future i'm going to try again (i still have like 8kg of oxides and a new battery i found a few weeks ago to process) and i'll write here
if what i'm doing is working
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Ubya  | | in the near future i'm going to try again (i still have like 8kg of oxides and a new battery i found a few weeks ago to process) and i'll write here
if what i'm doing is working |
Looking through the different methods to produce CO or H2, it seems that woodgas is probably the easiest way to make a reducing gas as there are lots
of web pages where people have made them from producing a L per minute to 50-100L/min to run small power plants.
I'm not sure what the temp needs to be for the reduction to work, I suspect the hotter the better and I was looking into using some cast iron pipe or
maybe some ferritic (non-austenitic stainless steel - 304 & 316 aren't magnetic) stainless - and heating the pipe from the outside with a torch or
maybe even induction b/c temp control might be easier.
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
Quote: Originally posted by RogueRose  | | Well, I'd appreciate if people stuck to the title, if you want to post where to find lead bullets, or buy it, there is a whole forum to post those in,
please feel free. But for the people who actually want to understand how it is done, then please feel free to discuss the process here. I'm not sure
everyone has access to a "limestone quarry filled with lead bullets" or a range that will sell lead (it's "hazardous" remember..). So there are
people who want to know how to do it from batteries, and considering 2-3 batteries equals what took cody all day panning for in a shooting range,
people might want to go that avenue. So, please no more derailing the topic. If you want to add to the original premise of the post, please go ahead.
Thanks |
when a Person about to do something hard and possibly dangerous to achieve the same goal when there is safe and much better ways around other people
should show them the right one.
since you asked,
there is lots of link and YT videos in this page http://www.sciencemadness.org/smwiki/index.php/Lead
[Edited on 26-9-2019 by rockyit98]
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
| Quote: |
when a Person about to do something hard and possibly dangerous to achieve the same goal when there is safe and much better ways around other people
should show them the right one.
|
You just posted a post about jet fuel from plastic, why not starting from petrol? It's easier.
Sometimes a simpler solution is not a better solution, not everyone has the same opportunities. Plus, lot's of people "recycle" car batteries for the
lead, but the yield is always low because the battery was discharged/broken, I don't like 99% of YouTube videos about recovering lead from batteries
because they just get the metallic lead, and discard the oxide (probably not in a safe way) where is most of the metal.
[Edited on 26-9-2019 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by rockyit98  | Quote: Originally posted by RogueRose  | | Well, I'd appreciate if people stuck to the title, if you want to post where to find lead bullets, or buy it, there is a whole forum to post those in,
please feel free. But for the people who actually want to understand how it is done, then please feel free to discuss the process here. I'm not sure
everyone has access to a "limestone quarry filled with lead bullets" or a range that will sell lead (it's "hazardous" remember..). So there are
people who want to know how to do it from batteries, and considering 2-3 batteries equals what took cody all day panning for in a shooting range,
people might want to go that avenue. So, please no more derailing the topic. If you want to add to the original premise of the post, please go ahead.
Thanks |
when a Person about to do something hard and possibly dangerous to achieve the same goal when there is safe and much better ways around other people
should show them the right one.
since you asked,
there is lots of link and YT videos in this page http://www.sciencemadness.org/smwiki/index.php/Lead
[Edited on 26-9-2019 by rockyit98] |
While I appreciate the saftey aspect, that wasn't the goal. Do you do the same for every thread in this forum? That's a pretty big job. I'm pretty
sure the thread title and following content was pretty specific as to the goal. If I had wanted to find a place online to buy or some other product
that contains lead, I'd probably have asked those questions instead.
As far as other YT video's and other threads, they don't cover full conversion very well and it seems there can be a VERY wide variance between
batteries in recovery and I suspect that has to do with the age/health of the battery and many of them that people try to recover are either heavily
sulfated or fully discharged leaving less pure lead to easily recover.
It's not an easy task but with the availability of batteries across the world, many people find that this is the best source and I think it's
worthwhile coming up with a way to recover the lead safely and reliably rather than have people making a mess and having to guess at temps, times, etc
in the process.
Looking at the recycling process it's clear that they deal with batteries "as-is" so they aren't charging them or desulfating them, just grinding and
separating plastic. From there the process varies based on the company where some use a chemical process and others use heat or heat/gas.
I know you haven't been here long but there are lots of threads where people do stuff because it's a challenge or because it's needed for some people.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by RogueRose  | Quote: Originally posted by Ubya  | | in the near future i'm going to try again (i still have like 8kg of oxides and a new battery i found a few weeks ago to process) and i'll write here
if what i'm doing is working |
Looking through the different methods to produce CO or H2, it seems that woodgas is probably the easiest way to make a reducing gas as there are lots
of web pages where people have made them from producing a L per minute to 50-100L/min to run small power plants.
I'm not sure what the temp needs to be for the reduction to work, I suspect the hotter the better and I was looking into using some cast iron pipe or
maybe some ferritic (non-austenitic stainless steel - 304 & 316 aren't magnetic) stainless - and heating the pipe from the outside with a torch or
maybe even induction b/c temp control might be easier. |
Hydrogen reduces lead oxide at temperatures below 200°C, while CO needs around 1000-1200°C. Maybe instead of producing woodgas you can make
watergas. Water gas is a mixture of CO and H2, produced by the reaction of steam with hot coal(or charcoal), so maybe a could modify my design by just
adding a small tube that brings water vapour to the hot combustion zone, CO and H2 are produced, and reduce the lead oxide while traveling up through
the furnace. The production of water gas is endothermic, but the fact that hydrogen needs lower temperatures to react with the oxide would make this
worthwhile.
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
RogueRose
International Hazard
    
Posts: 1595
Registered: 16-6-2014
Member Is Offline
|
|
Quote: Originally posted by Ubya  |
| Quote: |
when a Person about to do something hard and possibly dangerous to achieve the same goal when there is safe and much better ways around other people
should show them the right one.
|
You just posted a post about jet fuel from plastic, why not starting from petrol? It's easier.
Sometimes a simpler solution is not a better solution, not everyone has the same opportunities. Plus, lot's of people "recycle" car batteries for the
lead, but the yield is always low because the battery was discharged/broken, I don't like 99% of YouTube videos about recovering lead from batteries
because they just get the metallic lead, and discard the oxide (probably not in a safe way) where is most of the metal.
[Edited on 26-9-2019 by Ubya] |
Exactly. I've yet to see a comprehensive video and most all of them are pretty poor, like king of random, it has tons of views but is terrible in how
it shows what to expect or the process.
As far as your next process, are you going to try charging the battery first? Check the V before charging and then after and let it sit for a day or
two and see if/how much the V drops. If you can put a load across it, see if the V drops and by how much. It will give you an idea of the health of
the battery. If you don't know how to do this let me know and I'll explain more in depth. Also, if you can check the water level before charging,
that would be a good idea, add some DH2O if it needs it. I've seen batteries that had basically zero voltage come back to life after adding water.
IDK if they went dry over-charging or what.
Also, you should check to see how much the plates are sulfated. That would be the white substance on the negative plates and sometimes the positive
(PbO2) plates. The sulfate decomposes a little below 2000F, below the PbO melting point - so that is good I guess. Also it gives off SO3 as it
decomposes.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
About the battery, I tried to recover it when someone posted on the forum about reviving sulfated batteries, long story short, everything I tried
didn't work, so I just opened the top (it was a sealed battery) and saw the electrodes, they were deformed, crumbled, touching each other, extreme
physical damage, nothing could resuscitate that battery, reason why now I'm harvesting its lead.
[Edited on 26-9-2019 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
rockyit98
Hazard to Others
  
Posts: 283
Registered: 12-4-2019
Location: The Known Universe
Member Is Offline
Mood: no mood is a good mood
|
|
ok if that what you want
PbSO4 + Na2CO3---->PbCO3 +Na2SO4 The conversion of lead sulphate to lead carbonate in sodium carbonate media
https://sci-hub.tw/10.1016/0304-386X(92)90044-Z
PbCO3----(300 to 400C)------>PbO +CO2
2PbO + C →2Pb + CO2 (700C)
[Edited on 26-9-2019 by rockyit98]
"A mind is a terrible thing to lose"-Meisner
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Online
|
|
speculation;
PbO needs reduction to get Pb
the outer part of an air-hydrocarbon flame is oxidising,
the inner part of the flame is reducing,
so try using the inner part of the flame when aiming for Pb,
preferably use a yellow and/or smoky flame
in conjunction with your reducing agent (H2, C, CO etc.)
the flame should have excess hydrocarbon / incomplete combustion products
I'd expect the outer part of the flame to oxidise lead - which would be counter-productive.
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
| Pages:
1
2 |