| Pages:
1
2 |
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Ammonium formate from Ammonium chloride
Hey there,
So I need to get some ammonium formate for the Leuckart reaction, and the only ammonium source I've got is NH4Cl.
I'm guessing that NH4Cl won't react with HCOOH under STP condition..
But is there any easy to get catalysts/common reagents that might get the reaction going?
Also, is it possible to instead react NH4Cl with HCOONa? And if so, how would you separate it from the NaCl?
Much thanks! :)
[Edited on 25-9-2019 by B.D.E]
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
Mix the ammonium chloride with a strong base such as NaOH. Heat slightly or add a small amount of water. Bubble the evolved ammonia gas into the
HCOOH.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by hodges  | Mix the ammonium chloride with a strong base such as NaOH. Heat slightly or add a small amount of water. Bubble the evolved ammonia gas into the
HCOOH.
|
Thanks for the reply.
I thought about it, but I figured that the efficiency would be pretty low due to the poor kinetics of liquid-gas reactions. Was I wrong?
I can also get my hands on some formamide.
Is there a way to use it as a precursor of ammonium formate?
[Edited on 25-9-2019 by B.D.E]
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Quote: Originally posted by B.D.E  | Quote: Originally posted by hodges  | Mix the ammonium chloride with a strong base such as NaOH. Heat slightly or add a small amount of water. Bubble the evolved ammonia gas into the
HCOOH.
|
Thanks for the reply.
I thought about it, but I figured that the efficiency would be pretty low due to the poor kinetics of liquid-gas reactions. Was I wrong?
I can also get my hands on some formamide.
Is there a way to use it as a precursor of ammonium formate?
You can get formamide and you want to turn it into ammonium formate? That's kind of a backward step isn't it.you can use formamide in the leuckart you
know that right?
Or are you trying to use the ammonium formate directly? I've heard it works but whether or not it works better I can't say. |
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by draculic acid69  | Quote: Originally posted by B.D.E  | You can get formamide and you want to turn it into ammonium formate? That's kind of a backward step isn't it.you can use formamide in the leuckart you
know that right?
|
Or are you trying to use the ammonium formate directly? I've heard it works but whether or not it works better I can't say. |
I know that formamide can also be used as the Leuckart reagent, but from what I've read the yield is much lower compared to that obtained by the
ammonium formate method.
In addition, my ketone has some steric hindrance, which might make the formamide method even less efficient.
I saw in wikipedia(see attached picture) that the following reaction might take place:
HCONH2 + H2O --> HCOOH4N(I guess the rxn is in equilibrium?).
Is it really that simple? If anyone have some relevant data to share it would be swell 
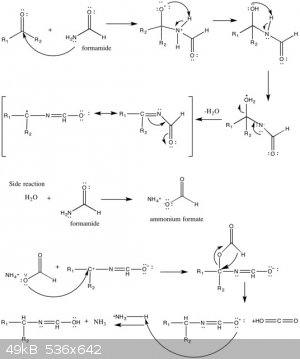
|
|
|
Pumukli
National Hazard
   
Posts: 708
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Hodges said it.
The absorbtion of ammonia will be 100%!
Poor kinetics of liquide-gas reactions?
Not in this case!
Ammonia likes water very much, but likes formic acid even more!
Actually I think your problem will be how to moderate the reaction, unless you want to end up with a thick, white, ammonium-formate smoke and dealing
with massive suck-back(s)!
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Yeah definitely suckback and thick rxn solution with product vapor being the only things that will kill yeild. If you use a coke bottle between your
nh3 generator and the formic acid it'll deal with any suckback and using a cold dilute solution will stop excessive product vapor and product
precipitating.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
You can always use a gas washing bottle to absorb the ammonia directly into ice-cold water to eliminate issues with reaction heat and viscosity. You
can easily get to >20wt% like this, and the resulting solution can then be carefully neutralized with formic acid.
Alternatviely, I have made acetamide by refluxing acetic acid with urea, and similar amides follow suit. Formamide should follow this formula, using
formic acid and urea. It takes a while but can be left largely unattended, and the reagents are dirt cheap which might work with the excess you need
for your Leukhart over the formate. Just a thought.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Volatility
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Thanks for the new information everyone! 
I'll probably go with the "bubbling ammonia method".
But they're both solids.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
NH4Cl was made by sublimation and it has been said that the formate can be distilled with vacuum...what happens when ammonium formate is distilled
without vacuum?
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
It decomposes to formamide and water.
[Edited on 2019-9-26 by Metacelsus]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Of course it does, so it requires vacuum fractionation next. Small amounts of various cpds. are known to increase yields when using the dry result.
[Edited on 27-9-2019 by S.C. Wack]
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
UPDATE:
Well.. I tried bubbling ammonia gas trough formic acid solution(<99%) and I got jack shit :S.
This is what i've tried:
>In an erlenmeyer flask I mixed NH4Cl&NaOH in a mol/mol ratio of 1:1(at this point without any water present).
>To the erlenmeyer I've connected a "vacuum takeoff" adapter with a rubber hose and a glass funnel at the end of it.
>I placed the funnel inside a beaker full of cold formic acid solution(99%, 1/2 eq). The beaker was placed into a cold water bath.
>I added ~10ml of non distilled water to the erlenmeyer and turned on the heating. The reaction started after a few minutes and than gradually got
more and more vigorous(it never got too crazy though). I lifted the funnel from the solution(just for a few seconds) and a white "fog" had appeared(as
expected).
The bubbling stopped after about ~15-20 minutes, and suck-back have occurred(the upside down funnel did its job). Almost no ammonium formate was
precipitate in the beaker. In addition, there were still a lot of powder left in the erlenmeyer. probably meaning not all the NH4Cl have reacted.
I've tried the all thing again with the same amounts of reagents(1:1:~0.5*), but this time with a more water(also none distilled). *I used the same solution of formic acid from the first try.
After bubbling stopped, it didn't seemed as if more ammonium formate had participated, and like the first try - there were still a lot of powder left
in the erlenmeyer(this time there was also a strong smell of ammonia coming from the erlenmeyer.
[Edited on 14-11-2019 by B.D.E]
|
|
|
TheMrbunGee
Hazard to Others
  
Posts: 364
Registered: 13-7-2016
Location: EU
Member Is Offline
Mood: Phosphorising
|
|
Quote: Originally posted by B.D.E  | UPDATE:
Well.. I tried bubbling ammonia gas trough formic acid solution(<99%) and I got jack shit :S.
This is what i've tried:
>In an erlenmeyer flask I mixed NH4Cl&NaOH in a mol/mol ratio of 1:1(at this point without any water present).
>To the erlenmeyer I've connected a "vacuum takeoff" adapter with a rubber hose and a glass funnel at the end of it.
>I placed the funnel inside a beaker full of cold formic acid solution(99%, 1/2 eq). The beaker was placed into a cold water bath.
>I added ~10ml of non distilled water to the erlenmeyer and turned on the heating. The reaction started after a few minutes and than gradually got
more and more vigorous(it never got too crazy though). I lifted the funnel from the solution(just for a few seconds) and a white "fog" had appeared(as
expected).
The bubbling stopped after about ~15-20 minutes, and suck-back have occurred(the upside down funnel did its job). Almost no ammonium formate was
precipitate in the beaker. In addition, there were still a lot of powder left in the erlenmeyer. probably meaning not all the NH4Cl have reacted.
I've tried the all thing again with the same amounts of reagents(1:1:~0.5*), but this time with a more water(also none distilled). *I used the same solution of formic acid from the first try.
After bubbling stopped, it didn't seemed as if more ammonium formate had participated, and like the first try - there were still a lot of powder left
in the erlenmeyer(this time there was also a strong smell of ammonia coming from the erlenmeyer.
[Edited on 14-11-2019 by B.D.E] |
Ammonium formate Solubility in water
(grams per 100g of water)
102g (0 °C)
142.7 g (20 °C)
202.4 g (40 °C)
516 g (80 °C)
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I'm fairly sure that mixing solutions of sodium formate and ammonium chloride in wet methanol would produce a ppt of NaCl.
Might be interesting to try.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by unionised  | I'm fairly sure that mixing solutions of sodium formate and ammonium chloride in wet methanol would produce a ppt of NaCl.
Might be interesting to try. |
Very interesting idea. I think I'll try it, but with dry methanol and sodium formate instead. Hoping that the methanol-formate solution would be
enough to dissolve the NH4Cl. If not I will slowly add water to the mixture.
Thanks mate 
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Screw that shit. I'll just buy ammonium carbonte or urea(for NH3 gas generator). I spent way too much time on trying to use NH4Cl.
Quick report: First I tried to dissolve sodium formate in a dry methanol solution. It is soluble but only slightly(20gr of HCOONa didn't fully
dissolved in ~250ml of 50°C methanol, not even close). I tried adding dH2O slowly using a pipette but I ended up using something like ~150ml until
everything(HCOONa&NH4Cl) got dissolved(after heating it up).
At that point I realized the methanol doesn't have any real use due to the large amounts of water present. So I boiled it off and continued heating
the aqous solution to a boil. I boiled off large portion of the water and let the solution cool. a white ppt fell from the solution. I decanted the
leftover water and washed the white ppt twice with diethyl ether. NH4Cl&HCOONa are insoluble in ether, while HCOONH4&NaCl are soluble. I
distilled the ether to dryness but there weren't any significant amount of salts to be found(only a tiny bit, similar to what you get after boiling
off 100ml of tap-water).
If I were to repeat this process, I would have tried to reflux the solution for about 12 hours or so, to see if it will make any difference. Also I
might have used soxhelt extractor with the ether, in order to make sure I fully dissolved all the NaCl&HCOONH4 out of the ppt. But to be honest,
I'm not sure if it would work even than, and it just doesn't worth the time.
Keep in mind that it is also possible that I just fucked something up in the process, I'm still a beginner after all. But due to the simplicity of
the alleged reaction, I found it hard to believe I fucked things up so badly to a level I didn't got any measureable amount of NaCl&HCOONH4 out of
the ether...
Good night :S
|
|
|
mackolol
Hazard to Others
  
Posts: 459
Registered: 26-10-2017
Member Is Offline
Mood: Funky
|
|
Quote: Originally posted by B.D.E  | Screw that shit. I'll just buy ammonium carbonte or urea(for NH3 gas generator). I spent way too much time on trying to use NH4Cl.
I decanted the leftover water and washed the white ppt twice with diethyl ether. NH4Cl&HCOONa are insoluble in ether, while HCOONH4&NaCl are
soluble. I distilled the ether to dryness but there weren't any significant amount of salts to be found(only a tiny bit, similar to what you get after
boiling off 100ml of tap-water).
If I were to repeat this process, I would have tried to reflux the solution for about 12 hours or so, to see if it will make any difference. Also I
might have used soxhelt extractor with the ether, in order to make sure I fully dissolved all the NaCl&HCOONH4 out of the ppt. But to be honest,
I'm not sure if it would work even than, and it just doesn't worth the time.
Keep in mind that it is also possible that I just fucked something up in the process, I'm still a beginner after all. But due to the simplicity of
the alleged reaction, I found it hard to believe I fucked things up so badly to a level I didn't got any measureable amount of NaCl&HCOONH4 out of
the ether...
Good night :S |
I don't know where did you read this but no salt is soluble in ether. Thats biggest bullshit I have heard.
I think that just bubbling ammonia through formic acid will do the job but don't expect any precipitate to form. Just distill this solution off, under
vacuum for the best results until but be careful as ammonium formate decomposes at 180C and melts at 116C. Maybe better option will be just adding
acetone or some alcohol to the mother liquor and the salt should precipitate off then due to lower polarity of solvent.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Quote: Originally posted by mackolol  | | I think that just bubbling ammonia through formic acid will do the job but don't expect any precipitate to form. |
"The ammonium salt was prepared by passing ammonia into 90% formic acid. Towards the end of the absorption, the temperature was raised sufficiently to
ensure the deposition of the neutral (instead of the acid) salt. The product obtained was recrystallized from absolute alcohol and then desiccated
over 99%) sulfuric acid in vacuo. The melting point of the final material was 117.3C"
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by mackolol  |
I don't know where did you read this but no salt is soluble in ether. Thats biggest bullshit I have heard.
I think that just bubbling ammonia through formic acid will do the job but don't expect any precipitate to form. Just distill this solution off, under
vacuum for the best results until but be careful as ammonium formate decomposes at 180C and melts at 116C. Maybe better option will be just adding
acetone or some alcohol to the mother liquor and the salt should precipitate off then due to lower polarity of solvent. |
First of all,thanks for the reply and for the willing to help. I was surprised too about the ether solubility but I found a few sources clearly
stating that HCOONH4 is indeed soluble in ether:
http://chemister.ru/Database/properties-en.php?dbid=1&id...
http://www.intox.org/databank/documents/chemical/ammform/cie...
http://www.sciencemadness.org/smwiki/index.php/Ammonium_form...
(just to name a few)
As for NaCl I only found a few unverified sources, but I didn't looked into it too much because I didn't really cared about it. I would definitely
going to check it myself whenever I'll succeed in making some HCOONH4 myself.
As for your comment on the ammonia method, I actually a bit embarrassed for not thinking on the solubility of the HCOONH4 in formic acid... I mean I
did cooled it to just above the melting point of formic acid, but even then the solubility is extremely high... Unfortunally, just after reading your
post I've tried crushing out the solution I've made with >95% ethanol and nothing had precipitate. It was kind of a rushed testing with only few
ml(it's late at night and I don't want to get too much into it right now). I will probably try it again tomorrow or sometime during the week.
But I still doubt that I'll get anything significant out of it. Not because bubbling ammonia in formic acid is a bad method, but because I had
troubles liberating significant amounts of ammonia gas from ammonium chloride(as mentioned in a previous post in this thread).
I also saw that NurdRage just released a video on a similar issue he encountered, only in his case he used ammonium nitrate instead. He eventually
switched to urea for better yield of ammonia gas. That's why if I won't be able to get ammonium carbonate I will buy some urea instead(as also been
mentioned on the same previous post).
[Edited on 18-11-2019 by B.D.E]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
How do you generate ammonia from ammonium chloride? Mixing the chloride with excess NaOH and slowly adding water should do the job.
I wouldn't go for crashing out with lower alcohols here as ammonium formate is soluble in them.
I once prepared ammonium formate with concentrated ammonia and concentrated formic acid. This works fine but drying was a b*tch. If you have ether the
extraction of the formate from dry NH4Cl and sodium formate sounds interesting. You could try to stir a mixture in ether for some days.
[Edited on 19-11-2019 by Tsjerk]
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Quote: Originally posted by Tsjerk  | How do you generate ammonia from ammonium chloride? Mixing the chloride with excess NaOH and slowly adding water should do the job.
I wouldn't go for crashing out with lower alcohols here as ammonium formate is soluble in them.
I once prepared ammonium formate with concentrated ammonia and concentrated formic acid. This works fine but drying was a b*tch. If you have ether the
extraction of the formate from dry NH4Cl and sodium formate sounds interesting. You could try to stir a mixture in ether for some days.
[Edited on 19-11-2019 by Tsjerk] |
I tried exactly that, only with equivalent amount of NaOH. Later today I would try crushing out the solution with toluene. Hopfully I would get
something out of it.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Toluene will just give you a two phase system, nothing in your mixture is miscible with it.
|
|
|
B.D.E
Hazard to Self
 
Posts: 97
Registered: 5-8-2019
Member Is Offline
Mood: Oscillating
|
|
Yeah you're right. But weird enough I tried crushing the solution both with acetone and with ether(on separate tries), and both times I've gotten a
two layer system. I decided just pouring it to a crystalizing dish and leaving it for a while on minimal heating. I hope that by this time tommorow it
would be done evaporating and I'll be left with at least some ammonium formate. If not I'll probably be putting this on hold for a while.
Cheers.
[Edited on 19-11-2019 by B.D.E]
[Edited on 20-11-2019 by B.D.E]
|
|
|
| Pages:
1
2 |