TmNhRhMgBrSe
Hazard to Others
  
Posts: 112
Registered: 4-7-2019
Member Is Offline
|
|
Thiocyanate turned red
I made some new calcium nitrate solution but its slightly yellow. I added some of it to sodium thiocyanate solution. The solution turned deep red so I
think it has some iron. Is iron the only thing that can make thiocyanate turn red?
sorry for bad english
|
|
|
teodor
National Hazard
   
Posts: 922
Registered: 28-6-2019
Location: Netherlands
Member Is Offline
|
|
Not sure whether iron is the only thing to develop red color in the thiocyanate test but pretty sure that any not chemically pure sample of calcium
salt contains a lot of iron. I would try to remove the iron (see "Inorganic Preparations" Henderson/Fernelius, excercise 3) and repeat the test. Also
it is possible to remove iron by making complex with citric acid, once I made purification of Ca(OH)2 with this method but it requires quite a big
amount of the acid.
[Edited on 22-8-2019 by teodor]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Iron probably isn't the only thing that makes thiocyanate turn red.
But it is the thing that is the 4th commonest element in the Earth's crust.
|
|
|
Rhodanide
Hazard to Others
  
Posts: 348
Registered: 23-7-2015
Location: The 80s
Member Is Offline
Mood: That retro aesthetic
|
|
I think I know what's happening. You say you "made" Ca(NO3)2 solution? And I'm assuming you used a Ca salt and HNO3?
The red color would make sense then, because at low concentrations KSCN reacts with most mineral acids (HNO3 included) to form the deep red
Perthiocyanic acid. With concentrated HNO3, KSCN reacts violently to form orange, insoluble Perthiocyanogen/Polythiocyanogen and readily expels LOTS
of NO2. KSCN also can react with other acids such as HCl or H2SO4 to form Perthiocyanic acid, which again is a cherry
red color. Let me know if I'm close to correct about how you made that solution. :]
Here's a photo from a while back in which I tried making solid Perthiocyanic acid from KSCN + H2SO4. The chunks floating around
are snow to cool the reaction mixture.

|
|
|
Bedlasky
International Hazard
    
Posts: 1241
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
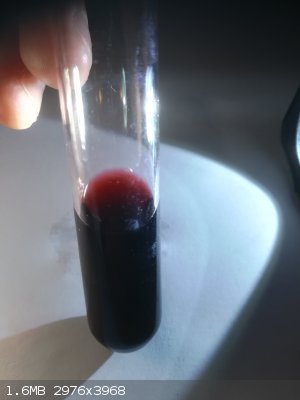
Only one interesting thing.
I recently made Mo(V)-isothiocyanate complex which is dark red too.
[Edited on 29-8-2019 by Bedlasky]
|
|
|
TmNhRhMgBrSe
Hazard to Others
  
Posts: 112
Registered: 4-7-2019
Member Is Offline
|
|
Quote: Originally posted by Rhodanide  | I think I know what's happening. You say you "made" Ca(NO3)2 solution? And I'm assuming you used a Ca salt and HNO3?
The red color would make sense then, because at low concentrations KSCN reacts with most mineral acids (HNO3 included) to form the deep red
Perthiocyanic acid. With concentrated HNO3, KSCN reacts violently to form orange, insoluble Perthiocyanogen/Polythiocyanogen and readily expels LOTS
of NO2. KSCN also can react with other acids such as HCl or H2SO4 to form Perthiocyanic acid, which again is a cherry
red color. Let me know if I'm close to correct about how you made that solution. :]
Here's a photo from a while back in which I tried making solid Perthiocyanic acid from KSCN + H2SO4. The chunks floating around
are snow to cool the reaction mixture.
|
I made Ca(NO3)2 from CaCO3 and excess HNO3. I neutralised remaining HNO3 with NaOH until some white
precipitate form. I filtered to remove the precipitate.
sorry for bad english
|
|
|