milovess
Permanently banned
Posts: 34
Registered: 19-12-2018
Member Is Offline
|
|
Does Cr from some salt displaces AL from AL2(SO4)3 ?
Hello Guys. I want to create Cr2(SO4)3 using AL2(SO4)3, but I'm not sure which anion is able to attract AL more than SO4^2- so please, if you know the
electronegativity order of anions suitable for the displacement, enlighten me 
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
If one uses Al2(SO4)3 in water so as to create a very dilute solution, the following reaction proceeds:
Al2(SO4)3 + 3 H2O = 2 Al(OH)3 (s) + 3 H2SO4
Source: Per Wkipedia (https://en.wikipedia.org/wiki/Aluminium_sulfate ) to quote:
"When dissolved in a large amount of neutral or slightly alkaline water, aluminium sulfate produces a gelatinous precipitate of aluminium hydroxide,
Al(OH)3."
Now, I would guess that in a galvanic cell (Cr metal, dilute H2O2, and say, some graphite rods, where the surface area of the latter carbon source at
least equals or exceeds the surface area of a solid piece of chromium metal and NaCl as an electrolyte), some chromium oxide may be formed via a
microwave assisted reaction. The question is whether dilute sulfuric acid created (via Al2(SO4)3 ) will react with the freshly created chromium oxide
in a MW assisted reaction, rich in radicals. Perhaps, as I have produced success for metals like copper with dilute 3% H2O2 and 5% vinegar in place of
current proposed dilute H2SO4.
Note, per the underlining chemistry, it is radicals created in the mix and, in this case, the actually very powerful sulfate radical anion
constructed, from the sulfate ion, which is driving the reaction products, and NOT the acid's pH at the start of the reaction. For more details, see,
for example, https://www.sciencemadness.org/whisper/viewthread.php?tid=15... .
Possible products include chromium sulfate and/or a basic sulfate.
If successful, please provide pictures.
Otherwise, per more standard procedures, the odds of success are low.
[Edited on 12-8-2019 by AJKOER]
|
|
|
Boffis
International Hazard
    
Posts: 1901
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
What form is your Cr in? Is is metallic chromium, if so it will not displace Al from its sulphate. This could be a very tricky conversion!
|
|
|
milovess
Permanently banned
Posts: 34
Registered: 19-12-2018
Member Is Offline
|
|
AJKOER
Thank you very much Bruh  I forgot to mention that the Cromium source I got is
CrO3. Unfortunatly your proposed method might not work since this will happen after diluted H2SO4 is able to react with my CrO3:---------
Cr2(SO4)3+AL(OH)3>AL2(SO4)3+Cr(OH)3 beacuse SO4^2- is more electronegative than OH^1- and attracts the least electronegative AL, and not Cr. It
appears on this table (its kinda cheap, since not all anions are included) that I might succeed to dispace AL from AL2(SO4)3 by adding CrF3 to
AL2(SO4)3. I would have defenetly tried your method if I had Cromium metal, but I'm too lazy to try reducing CrO3 to Cr metal by mixing it with
Graphite powder and applying high voltage and current to the mixture by shorting inside two Carbon electrodes. I was thinking to thermally decompose
AL2(SO4)3 into SO3 but I dont have suitable reactor yet, so I might instead do this with the Cr2(SO4)3 if I even create it: I forgot to mention that the Cromium source I got is
CrO3. Unfortunatly your proposed method might not work since this will happen after diluted H2SO4 is able to react with my CrO3:---------
Cr2(SO4)3+AL(OH)3>AL2(SO4)3+Cr(OH)3 beacuse SO4^2- is more electronegative than OH^1- and attracts the least electronegative AL, and not Cr. It
appears on this table (its kinda cheap, since not all anions are included) that I might succeed to dispace AL from AL2(SO4)3 by adding CrF3 to
AL2(SO4)3. I would have defenetly tried your method if I had Cromium metal, but I'm too lazy to try reducing CrO3 to Cr metal by mixing it with
Graphite powder and applying high voltage and current to the mixture by shorting inside two Carbon electrodes. I was thinking to thermally decompose
AL2(SO4)3 into SO3 but I dont have suitable reactor yet, so I might instead do this with the Cr2(SO4)3 if I even create it:
I saw this decomposition in electrochemistry book:
Cr2(SO4)3+3O+5H2O>2H2CrO4+3H2SO4, both of which are very usefull separatly, but I wonder what this mixture between H2CrO4 and H2SO4 might do to
Alcohol, whether it will create Ethanoic acid or diEththyl Sulfate? 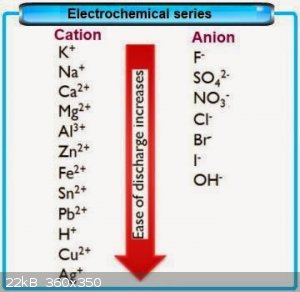
[Edited on 12-8-2019 by milovess]
[Edited on 12-8-2019 by milovess]
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Actual it may work due to the creation of the insoluble basic sulfate!
------------------------------------
With respect to Cr metal, or more precisely Cr plate, is visible on car parts. To quote a source (http://www.chem4kids.com/files/elements/024_speak.html ):
"Chrome! Shiny, reflective car bumpers and hubcaps."
and apparently grills.
A metal file is the best investment I ever made (and, interestingly, I was asked what I intended to use it for!).
--------------------------------------------------------
More on Aluminum sulfate per Wiki to quote in parts:
"Aluminium sulfate is used in water purification.... it causes suspended impurities to coagulate into larger particles and then settle to the bottom
of the container....Research suggests that in Australia, aluminium sulfate used this way in drinking water treatment is the primary source of hydrogen
sulfide gas in sanitary sewer systems.[8] An improper and excess application incident in 1988 polluted the water supply of Camelford in Cornwall."
OK, some pretty strong chemistry going on there (leading to the breakdown of the sulfate ion), which is apparently due to bacteria (see https://www.sciencedirect.com/topics/biochemistry-genetics-a... ).
[Edited on 13-8-2019 by AJKOER]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by milovess  | Hello Guys. I want to create Cr2(SO4)3 using AL2(SO4)3, but I'm not sure which anion is able to attract AL more than SO4^2- so please, if you know the
electronegativity order of anions suitable for the displacement, enlighten me 
|
The anion is almost completely irrelevant, unless it's something that coordinates strongly to chromium and not to aluminum. Chromium will not
displace aluminum from any compound that I can think of, or in any solvent that I can think of (except possibly molten cyanides, which you'd be crazy
to try to use).
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
milovess
Permanently banned
Posts: 34
Registered: 19-12-2018
Member Is Offline
|
|
DraconicAcid
If the table I posted is not fake, then AL from AL2(SO4)3 will be able to displace Cr from CrF3 considering that F^1- anion is more electronegative
than SO4^2-
|
|
|
woelen
Super Administrator
        
Posts: 8084
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
You are mixing up metallic species and ionic (already oxidized) species of metals. The Al in Al2(SO4)3 is not available as reductor, it already is
oxidized and cannot replace Cr from any salt. It makes no sense to apply the electrochemical series in this situation. It is like using a hammer to
solder electronic parts 
If you add metallic Al to a solution of a Cr-salt, then you might have conversion to Cr metal (and the Al going into solution as 3+ ion).
Making Cr2(SO4)3 from Al2(SO4)3 is not feasible. You might be able to make mix-crystals by adding another Cr(3+) salt to a solution of Al2(So4)3 and
then allowing the liquid to evaporate. Cr(3+) can replace Al(3+) in certain crystal lattices, without disturbing the overall shape of the crystals.
This is used for making beautiful alum-crystals with a nice purple color, in which a small fraction of Al(3+) ions is replaced by Cr(3+) ions.
|
|
|
Boffis
International Hazard
    
Posts: 1901
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
The issue with the electropositivity scale above is that you will almost certainly be working in water and therefore everything will be buffered by
hydrogen and oxygen and there aqueous ions.
Is you are starting with CrO3 then can you get a metabisulphite salt, either sodium or potassium. If so you can either mix them with the chromic oxide
in solution directly to get sodium (or potassium) chromium III sulphate or you can mix your aluminium sulphate with the metabisulphite and heat to
drive off the SO2 and bubble this through the chromic oxide solution until it is blue green throughout (may be violet if you can keep it cool).
Aluminium sulphate does not form a stable sulphite so on heating you will drive off SO2 from a mixture of its solution and a strong solution
metabsulphite. It would be better to use an acid such as hydrochloric acid or sulphuric acid though to generate the SO2. The gaseous SO2 method
ensures you end up with just chromium III sulpahte in solution.
|
|
|
milovess
Permanently banned
Posts: 34
Registered: 19-12-2018
Member Is Offline
|
|
woelen
I will disagree. Its seems like you never heard about double displacement reaction...... Pity you! Here behold and do some reading Bruh: https://en.m.wikipedia.org/wiki/Salt_metathesis_reaction
If you're too lazy here are examples of it:
NH4NO3+NaCL>NaNO3+NH4NO3
................
Ba(OH)2 + 2CuCNS → Ba(CNS)
2 + 2CuOH
..............

|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
To be clear on my comments, the starting materials are Cr metal and Al(OH)3 (a precipitate) and SO4(2-) ions. The action of microwave on graphite
leads to electrons on the carbon surface and also solvated electrons (e-(aq)). The latter can interact with, for example, the dilute H2O2 to form yet
more radicals, like .OH, which can further react with SO4(2-) to create .SO4- (the sulfate radical radical anion) and therefrom sulfate (and more)
salts.
.SO4- + .SO4- = S2O4(2-)
.SO4- + Cr(2+) --> SO4(2-) + Cr(3+) (See Reaction 2.10 on page 15 at https://nvlpubs.nist.gov/nistpubs/Legacy/NSRDS/nbsnsrds65.pd... )
The action of e-(aq) with.OH leads also to OH-. As such, my best guess is that in the presence of metal Cr, final products may include, to a limited
degree, a visible precipitate of a basic chromium sulfate.
Note, per the possible electrochemical reaction (which, per my recollection is certainly valid for Fe, Cu, Mn and Co):
Cr + O2 + 2 H+ --> Cr(2+) + 2 OH-
an intermediate Cr(OH)2 may form to further react. The above is also the basis for the creation of select basic salts. For example, with copper, see
discussion and references at https://www.sciencemadness.org/whisper/viewthread.php?tid=14... .
One can overlay on the above some standard chemical reactions if you so desire, but I believe that an obvious precipitate may develop other than
Al(OH)3, perhaps even a basic phosphate if the H2O2 contained added H3PO4.
[Edited on 13-8-2019 by AJKOER]
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
and you probably never heard of equilibriums, those reactions work because some species precipitate or is freed as a gas, this way the equilibrium is
shifted to the products (Le Chatelier principle, if you remove a product, more reagents will react).
adding cromium metal or a cromium salt to aluminium sulfate doesn't generate a precipitate, you could get one to precipitate lowering the temperature
if they have different solubilities (the same way you get potassioum dichromate from the sodium salt because the potassium salt is waaay less soluble)
but this is not the case as they have pretty close solubilities
[Edited on 13-8-2019 by Ubya]
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by milovess  | | If the table I posted is not fake, then AL from AL2(SO4)3 will be able to displace Cr from CrF3 considering that F^1- anion is more electronegative
than SO4^2- |
The table isn't fake- you're just completely misreading it.
The table says that fluorine will displace chlorine from a compound in a single replacement reaction. The reaction 2 AlCl3 + 3 F2 -> 2 AlF3 + 3
Cl2 is perfectly feasible; the reverse reaction will not occur.
It also says that aluminum is more reactive than chromium, so the reaction Al + CrCl3 --> Cr + AlCl3 is feasible (although possibly not in aqueous
solution); the reverse is not.
These are SINGLE replacement reactions- you've got an element reacting with a compound.
It does not say ANYTHING about double replacement reactions. The reactivities, reduction potentials, and electronegativities will have absolutely no
effect upon whether a reaction such as CrCl3 + AlF3 --> CrF3 + AlCl3 will proceed. For a metathesis reaction, the reaction will proceed if one of
the products is stabilized more than the reactants (forming an insoluble precipitate, forming a non-electrolyte forming a gas that removes itself from
solution, etc). Not going to be the case here.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
woelen
Super Administrator
        
Posts: 8084
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Unfortunately you show that your understanding of the theory is lacking completely. Now you are talking about double displacement, in your previous
post you were talking about electrochemical series.
I can tell you (and others already did this as well, in other words) that double displacement has nothing to do with redox reactions, so applying an
electrochemical series for that really does not make sense. Read DraconicAcid's answer. That answer explains things quite well.
Single replacement reactions as mentioned by DraconicAcid are redox reactions. The oxidation state of one element increases, while the oxidation state
of another element decreases, e.g. Al + Cr(3+) --> Al(3+) + Cr, which is feasible under the right conditions (e.g. in a thermite-like mix).
Another example is the following: 2 Fe(2+) + Cl2 --> 2 Fe(3+) + 2 Cl(-). The latter is not a real replacement reaction (both products go into
solution), but it is a nice example of another redox reaction, which very easily occurs in practice, which is also confirmed by the relative positions
of Fe(2+) ion and elemental chlorine in the electrochemical series. In both examples you have an increase of oxidation state of one element and a
corresponding decrease of oxidation state of another element:
example 1: from 0 to +3 for Al; from +3 to 0 for Cr.
example 2: from +2 to +3 for Fe; from 0 to -1 for Cl.
|
|
|
milovess
Permanently banned
Posts: 34
Registered: 19-12-2018
Member Is Offline
|
|
Ubya, DraconicAcid, AJKOER, woelen, Boffis
First of all, thanks to all of you for writing me back  , but I still see
contradition to your claims that cation displacement has nothing to do with electronegativity. , but I still see
contradition to your claims that cation displacement has nothing to do with electronegativity.
In a video about making NaNO3, Nurdrage did this: NaCL(aq)+NH4NO3(aq)>NaNO3(aq)+NH4CL(aq)
............ He then cooled down the mixture and said the formed cryatals at the bottom were NaNO3. If they were NaNO3, it will ONLY mean that Na^1+
displaced NH4^1- from NH4NO3. If you look at that table NO3^1- has higher negativity than CL^-1 and Na^1+ alegedly has lower negativity than NH4^1+
and the less negative bonds with the most negative from the mixture. By that logic it seems to me that the displacement works and is ruled solely on
electronegativity. That being said, I still dont feel debunked, but I appreciate your routes for synthesis of Cr2(SO4)3. Furthermore, I'll try this as
soon as I get a fluoride source: 2CrF3+AL2(SO4)3>Cr2(SO4)3+2ALF3. If H2O is problematic due to close solubilities of the aleged products, how about
using polar aprotic solvent like CH3OCH2CH3 or CH3(CO)CH3 ? Do you think their solubilities will differ a lot more to each other ?
[Edited on 14-8-2019 by milovess]
[Edited on 14-8-2019 by milovess]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
the electrochemical series chart you give is a little oversimplified. Something to remember is that electrochemical potential doesn't "belong" to an
ion by itself, like Na+, but to a particular half-reaction which includes that ion, like Na -> Na+ + e-. Sometimes it might make sense to report
the electronegativity of a single ion if it's obvious what the half-reaction is, but it's not obvious to me what the half-reaction would be for
ammonium ion. I don't think I have ever seen NH4+ on an electromotive chart, and it isn't included in yours, so I don't know who is alleging its order
relative to Na+.
The chart also lists calcium as less reducing than sodium, but that doesn't appear to be the case:
https://chem.libretexts.org/Under_Construction/Redox_Equilib...
So consider silver nitrate. (silver is below sodium on your chart) Add either sodium hydroxide (OH- is below nitrate on your chart) or sodium sulfate
(SO4 2- is above it) and a low-solubility silver salt will precipitate.
Now consider calcium nitrate (calcium is above sodium). Add NaOH or Na2SO4 and a low-solubility calcium salt will precipitate.
Finally ... think about Nurdrage's crystallization. If
NaCl(aq)+NH4NO3(aq)>NaNO3(aq)+NH4Cl(aq)
is driven by electronegativity, rather than solubility, why cool the reaction mixture?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by milovess  | First of all, thanks to all of you for writing me back  , but I still see
contradition to your claims that cation displacement has nothing to do with electronegativity. , but I still see
contradition to your claims that cation displacement has nothing to do with electronegativity.
In a video about making NaNO3, Nurdrage did this: NaCL(aq)+NH4NO3(aq)>NaNO3(aq)+NH4CL(aq)
............ He then cooled down the mixture and said the formed cryatals at the bottom were NaNO3. If they were NaNO3, it will ONLY mean that Na^1+
displaced NH4^1- from NH4NO3. If you look at that table NO3^1- has higher negativity than CL^-1 and Na^1+ alegedly has lower negativity than NH4^1+
and the less negative bonds with the most negative from the mixture. By that logic it seems to me that the displacement works and is ruled solely on
electronegativity. |
In that example, nothing is displacing anything from anything. If you dissolve a simple ionic compound in water, you get ions. The cations go off
and play with the water molecules, the anions go off and play with the water molecules, and the cations and anions really pay no attention to each
other.
If you evaporate some of the solvent and/or cool it down, the least soluble cation/anion combination will precipitate first. In this case, it's
sodium nitrate, not because of any electronegativity values, but just because the ions match in size better than the other combinations.
If you are sure that it's electronegativity, how do you explain that potassium chloride and potassium fluoride are perfectly soluble, but potassium
perchlorate and potassium hydrogen tartrate have very low solubilities? Do you think that the hydrogen tartrate ion is more electronegative than
fluorine? Or that perchlorate is less electronegative than chlorine?
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by milovess  |
In a video about making NaNO3, Nurdrage did this: NaCL(aq)+NH4NO3(aq)>NaNO3(aq)+NH4CL(aq)
............ He then cooled down the mixture and said the formed cryatals at the bottom were NaNO3. |
as i said, and as the others said, at low temperature sodium nitrate has a lower solubility than sodium chloride, so it will precipitate. in solution
you don't have NaCl, NH4NO3, you have Na+, Cl-, NH4+, NO3-, they are ionic compounds, at lower temperatures sodium nitrate crystallizes, removing Na+
and NO3- from solution, the same would happen if the reaction produced a precipitate or a gas.
you are still not convinced, i see, consider this, cesium metal can be distilled off a mixture of cesium chloride and calcium metal, calcium is lower
in the reduction series, so it should not be able to swap the cesium, but low and behold, it does at high temperatures, because cesium has a lower
boiling point than calcium, so it get distilled, and calcium takes its place in the chloride. or the same example i did before but you did not read,
adding potassium chloride to sodium dichromate makes a precipitate of potassium dichromate, following your theory this should not be possible as
potassium has a higher reduction potential (even though i can't understand the reasoning behind substitutions and reduction potentials as clearly
there is not a reduction/oxidation in a salt metathesis)
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
woelen
Super Administrator
        
Posts: 8084
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
Just another example to make things clear.
In a clean and dry bottle A someone puts exactly 1 mole of NaCl and 1 mole of KBr. Next, he dissolves all of it in water and then tops up the water to
exactly 1 liter while stirring.
In a clean and dry bottle B someone puts exactly 1 mole of KCl and 1 mole of NaBr. Next, he dissolves all of it in water and then tops up the water to
exactly 1 liter while stirring.
You can witness the person doing this and know in which bottle there is NaCl+KBr and in which bottle there is KCl+NaBr
Next he takes a sample from bottle A and a sample from bottle B and gives these to you. You could not see which sample is taken from which bottle. Can
you distinguish the two samples from each other by means of certain chemical analytical procedures and tell which sample is from which bottle?
|
|
|
chornedsnorkack
National Hazard
   
Posts: 566
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Elementary question:
do Al2(SO4)3 and Cr2(SO4)3 precipitate as separate crystals or as a solid solution of both?
|
|
|
DraconicAcid
International Hazard
    
Posts: 4416
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
I would assume the solution, as you would get in the alum.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
chornedsnorkack
National Hazard
   
Posts: 566
Registered: 16-2-2012
Member Is Offline
Mood: No Mood
|
|
Since Al is a nuisance to separate from Cr, why do you need it as a sulphate source?
Starting from CrO3, could you have
2CrO3+3SO2=Cr2(SO4)3?
|
|
|