thors.lab
Hazard to Others
  
Posts: 103
Registered: 19-7-2019
Member Is Offline
|
|
Reduction of nitrobenzene by zinc and ammonium chloride
Hi,
I've been studying the following reaction:
PhNO3 + Zn + NH4Cl --> Ph-NH-OH + ZnO + ?
I can't find much information on this. In particular, I'm looking for a reaction mechanism. I can't seem to find any anywhere. What role does the
ammonium chloride play in this reaction? Why ammonium chloride in particular, and why zinc in particular?
Thanks,
Thor
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
Vogel's Textbook of Practical Organic Chemistry:
| Quote: | | The reduction of an aromatic nitro compound with a powerful reducing agent (tin or tin(II) chloride and hydrochloric acid; iron and dilute
hydrochloric acid; hydrogen and a platinum catalyst) leads to a good yield of primary amine, e.g. aniline from nitrobenzene. By the use of
milder reducing agents and by the control of the hydrogen ion concentration of the solution, a number of intermediate products may be
isolated, some of which are products of direct reduction and others are formed through secondary reactions. |
Zn and NH4Cl can be replaced by other reducing agents/solvents.
http://orgsyn.org/demo.aspx?prep=CV1P0445
Mechanism (I'm looking for a reputable source):
https://labmonk.com/synthesis-of-p-aminophenol-from-nitroben...

[Edited on 31-7-2019 by icelake]
[Edited on 31-7-2019 by icelake]
|
|
|
thors.lab
Hazard to Others
  
Posts: 103
Registered: 19-7-2019
Member Is Offline
|
|
Quote: Originally posted by icelake  | Vogel's Textbook of Practical Organic Chemistry:
| Quote: | | The reduction of an aromatic nitro compound with a powerful reducing agent (tin or tin(II) chloride and hydrochloric acid; iron and dilute
hydrochloric acid; hydrogen and a platinum catalyst) leads to a good yield of primary amine, e.g. aniline from nitrobenzene. By the use of
milder reducing agents and by the control of the hydrogen ion concentration of the solution, a number of intermediate products may be
isolated, some of which are products of direct reduction and others are formed through secondary reactions. |
Zn and NH4Cl can be replaced by other reducing agents/solvents.
http://orgsyn.org/demo.aspx?prep=CV1P0445
Mechanism (I'm looking for a reputable source):
https://labmonk.com/synthesis-of-p-aminophenol-from-nitroben...
[Edited on 31-7-2019 by icelake]
[Edited on 31-7-2019 by icelake] |
I know somebody would cite Vogel's! Unfortunately I don't have a searchable copy of it so I couldn't find the reaction in it, but it did cross my
mind…
Unfortunately, I don't think that labmonk reaction is correct (or at least complete). Empirical evidence shows that Zinc Oxide is formed, while
labmonk fails to show this. Does water or the hydroxide ion then attack the zinc cation, and lose its hydrogen(s) to ammonia/water, reforming ammonium
chloride?
So ammonium chloride is just used in this reaction as a weak acid to "control the hydrogen ion concentration," while zinc functions as the weak
reducing agent?
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
I don't want to speculate about the formation of ZnO/ZnCl2/NH3 or the mechanism - I'll update this thread if/when I found a
definitive answer.
Quote: Originally posted by thors.lab  |
So ammonium chloride is just used in this reaction as a weak acid to "control the hydrogen ion concentration," while zinc functions as the weak
reducing agent? |
Bingo!
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Bo...
Reduction of nitro compounds occurs readily with a variety of reducing agents and such reductions afford a particularly useful synthesis of aromatic
amines (Section 23-12B):

The reduction of a nitro compound to an amine requires six equivalents of reducing agent:
R−NO2+6H⊕+6e⊖→RNH2+2H2O(24.6.7)
One would not expect such a reduction to occur in a single step. Indeed, reduction is stepwise and proceeds through a string of intermediates, which,
with strong reducing agents in acid solution, have at most a transient existence. The intermediates formed successively from RNO2by increments of two
equivalents of reducing agent are nitroso compounds, R−N=O, and N-substituted azanols (hydroxylamines), RNHOH:
RNO2⟶2[H]−H2ORN=O⟶2[H]RNHOH⟶2[H]−H2ORNH2(24.6.8)
Thus N-aryl-substituted azanols can be obtained directly from the corresponding nitro compounds with zinc and ammonium
chloride solution. However, zinc and hydrochloric acid gives the amine:

The difference between these reactions is in the reduction rates associated with the acidity of the solution. Ammonium
chloride is a much weaker acid than HCl; the pH of ammonium chloride solutions is around 6.
https://www.sciencedirect.com/science/article/pii/B978008011...
Phenylhydroxylamine may be obtained in good yield by reduction of nitrobenzene in neutral solution. Zinc dust or aluminium amalgam in water may be
used as the reducing agents. Ammonium chloride is used as a buffer in order to avoid the solution becoming too alkaline.
[Edited on 1-8-2019 by icelake]
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
A plausible mechanism according to Experimental Organic Chemistry: A Miniscale and Microscale Approach (page 707) - Zn or Sn, it shouldn't matter.
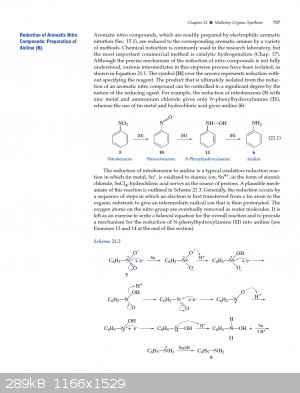
Attachment: 707.pdf (327kB)
This file has been downloaded 427 times
|
|
|
thors.lab
Hazard to Others
  
Posts: 103
Registered: 19-7-2019
Member Is Offline
|
|
Quote: Originally posted by icelake  | A plausible mechanism according to Experimental Organic Chemistry: A Miniscale and Microscale Approach (page 707) - Zn or Sn, it shouldn't matter.
|
Very nice find. Is tin stronger than zinc? Will it over-reduce the nitrobenzene past phenylhydroxylamine to aniline? Or will the use of ammonium
chloride rather than HCl effectively control the reduction?
|
|
|
icelake
Harmless

Posts: 48
Registered: 9-10-2017
Member Is Offline
Mood: No Mood
|
|
Zinc/Iron/Tin are fully capable of reducing the nitrobenzene to aniline (in acidic environment).
| Quote: | | will the use of ammonium chloride rather than HCl effectively control the reduction? |
Sn doesn't react that way, you'll need to use Sn/HCl or SnCl2 in order to reduce an aromatic nitro group - and AFAIK, Sn doesn't dissolve
in NH4Cl.
Forgot to mention this piece (Competition Science Vision) - I'm not quite sure what to make of reaction products (especially the ZnCl2).

[Edited on 4-8-2019 by icelake]
[Edited on 4-8-2019 by icelake]
|
|
|