Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Nitration of 2-EH
Hello World,
Usually I do the nitration of 2-EH by slowly adding 2-ethylhexanol into the mixed acid (MA) (so the acid is in
excess). The reaction takes place in a batch reactor (three neck flask) and I make sure that the temperature of the process is maintained
below 20*C. It is a fairly standard procedure. After the addition of 2EH is finished the acids are separated from the final product (2-ethylhexyl
nitrate; 2EHN). Next is the washing process: 3-4 x with water till the pH of the washing water is about 7 (usually after the first washing the water
is intense yellow in colour and after the last washing step it is colorless).
Yesterday I tried to do the nitration of 2-ethylhexanol by slowly adding the MA into the 2EH. So during the reaction the
alcohol was in excess. Close to the end of the addition the acids, the reacting mixture darkend, i.e. it turned light yellow-gray
(dirty pale yellow) in colour. After the addition I quenched the reaction by pouring the mixture into ice cold water. And then... First the organic
layer turned light green and started to bubbling like carbonated water and after few seconds it turned light blue. I separated the spent acids form
the product and washed it with distilled water, The product turned vivid light blue (photo attached). After the second washing step (also with water)
it changed its color to vivid light green (photo attached although it looks much darker than it really is).
 
When I added few drops of water KOH solution into a sample in test tube, the product turned blue again and the water- which separated from the mixture
- turned yellow.
It is hard to find any pattern in this, because the crude acidic product was blue. After the first washing, when most of the remaining inorganic acids
were washed out, it turned green. And in contact with potassium hydroxide it turned blue again...
After adding few drops of 96% H2SO4 the product turned yellow, then colorless and then it started to decompose (nitric oxides/ runaway reaction).
I checked it on FTIR and it is definitely a mixture of 2EHN and some other ester. Also it has a high bromine number ( >6 compared to <0.7 in
case of pure 2EHN) and a very pleasant fruity/ floral smell.
Next I ran the GC/MS analysis and the main product was 2EHN. The second most abundant compound was proposed by software to be 2-Ethylhexyl
formate...??
I repeated the process today because I thought that maybe the colors were due some contamination of the glass or something, but the results were the
same.
Can any of You shed some light on what compounds/ reactions may be responsible for these colors? Any ideas?
Regards,
Bender
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Nitroso compounds, nitrite esters? I've seen them blue and green and would not be suprised to see them decompose with acid.
[Edited on 23-3-2019 by Sigmatropic]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
2-ethylhexylnitrite seems plausible. It might have a GC/MS a little bit like the formate (NO and CHO have similar MW) and it makes sense that it would
form if alcohol is in excess and HNO3 gets reduced. Also its bromine number should be high if it reacts with bromine to form NOBr + alkyl hypobromite
which then dehydrohalogenates to a ketone that reacts with five moles of Br+
|
|
|
Bender84
Harmless

Posts: 32
Registered: 24-3-2016
Member Is Offline
Mood: No Mood
|
|
Thanks!
I'll check the product for 2-ethylhexylnitrite.
Also below you'll find the FTIR spectra.
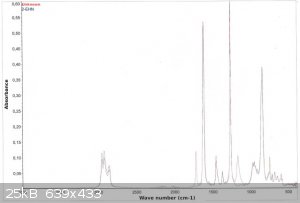
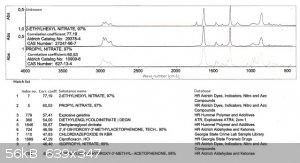
As you can see the product differs from the 2EHN by two bands: one at 1750-1780 cm-1 and the second at approx. 1180 cm-1 (in the fingerprint region).
The 1750-1780 band is most probably due to the C=O stretch.
Cheers!
|
|
|
otonel
Hazard to Self
 
Posts: 84
Registered: 9-4-2005
Member Is Offline
Mood: No Mood
|
|
I want to synthesis 2-ETHYLHEXYL NITRATE in large quantities to use as cetane improver in diesel fuel.
What is the best and cheap way to make this stuff?
[Edited on 2-6-2019 by otonel]
|
|
|