Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
cumene, cumol, isopropylbenzene....
Thats what I am after and can´t find it.
Looks like this:
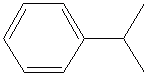
molfile
(CHIME plugin requireddownload)
The compound is produced in unbelievable amounts (about 7million tons a year), mostly to produce acetone and phenol and not more regulated than xylene
- but not to get at least for me. It is told to be still in use as additiv for airplane engines - thats left for me to look up. Even my chemical
supplier would have to order it what says a lot of paperwork and hassle and time.
So if anyone knows a OTC product which is international available or has another idea - welcome.
Also it may sound absurde: A easy way to add a methylgroup to toluene anyone? On the alpha carbon, not on the ring of course. Just adding
formaldehyde and some magic, would this work? (explicite description of needed magic please)
To go from toluene -> benzene -> cumene with two Friedl-Crafts and a unhealty compound as intermediate (benzene) seems to perverted even for me.

ORG 
! signature under construction !
[Edited on 21-5-2003 by Organikum]
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
Styrene could be a convienient starting point. It can be bought from fibreglass suppliers.
Reacting this with hydrogen bromide should give 1-bromoethylbenzene which can then be alkylated to give isopropylbenzene as required.
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
cumene, a replacement for n,n-dimethylaniline ?
Cumene is a useful buffer substance or chemical
for the manufacture of a tetra- or pentanitrate
similar than tetryl and pentryl.
 
In convention with my ancestor.
|
|
|
jimwig
Hazard to Others
  
Posts: 215
Registered: 17-5-2003
Location: the sunny south
Member Is Offline
Mood: No Mood
|
|
May I ask you a question? (without incurring an insult either way)
If you have access to references I will be glad to do some work toward published references on this synth.
I am not a chemist. Only an amateur so I say and do things that are very vulnerable to attack. I don't mind as long as I stand to gain in
knowledge from such.
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Dear fish, but thats not easier as toluene -> benzene -> cumene, and the styrene is still more hassle to aquire as toluene (xylene should also
work in the reverse Friedel-Crafts).
Jimwig, if you say you´re a simple amateur what am I then? Whats five steps below a simple amateur? Oh shit.....
And whom could you have offended how?
(btw. to what reaction do you refer? Toluene methylation or styrene bromination + alkylation?)
To many questions.
Good night.
addon: found a answer: Yes Jimwig ask as many questions you want! Thats the hidden deeper sense of this board I believe.
I hope I haven´t disclosed a secret now.....  - ah, no - the secret was that the
board is part of Polverones plan for world domination.... - ah, no - the secret was that the
board is part of Polverones plan for world domination.... 
[Edited on 21-5-2003 by Organikum]
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
| Quote: | Originally posted by Organikum
Dear fish, but thats not easier as toluene -> benzene -> cumene, and the styrene is still more hassle to aquire as toluene (xylene should also
work in the reverse Friedel-Crafts).
|
Only if you have a reflux still to perform the reverse Friedel-Crafts reaction.
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
reflux still?
Is that the same as a fractionating column? A vigreux won´t sufficice I believe, but a reasonable Hempel should do it! One like this:
 
On top belongs a stillhead with condensor, but thats only seldom necessary. The distillate should be redistilled anyways.
This opportunity to brag with one of the better pieces of my glassware I cannot let go... 
Hope you understand and forgive.
thanks
ORG 
btw. any usable tube filled with stainless steel pot-cleaners or a neontube with shards of glass works fine for most reactions. And is easy and
cheap.
[Edited on 22-5-2003 by Organikum]
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
| Quote: | This opportunity to brag with one of the better pieces of my glassware I cannot let go...  |
You know what they say about men who show of their expensive fractionation columns... 
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Yes it´s actually boring and hard to walk when everbody wants to kiss your feet. Not to talk of the immoral suggestions the girls make.... 
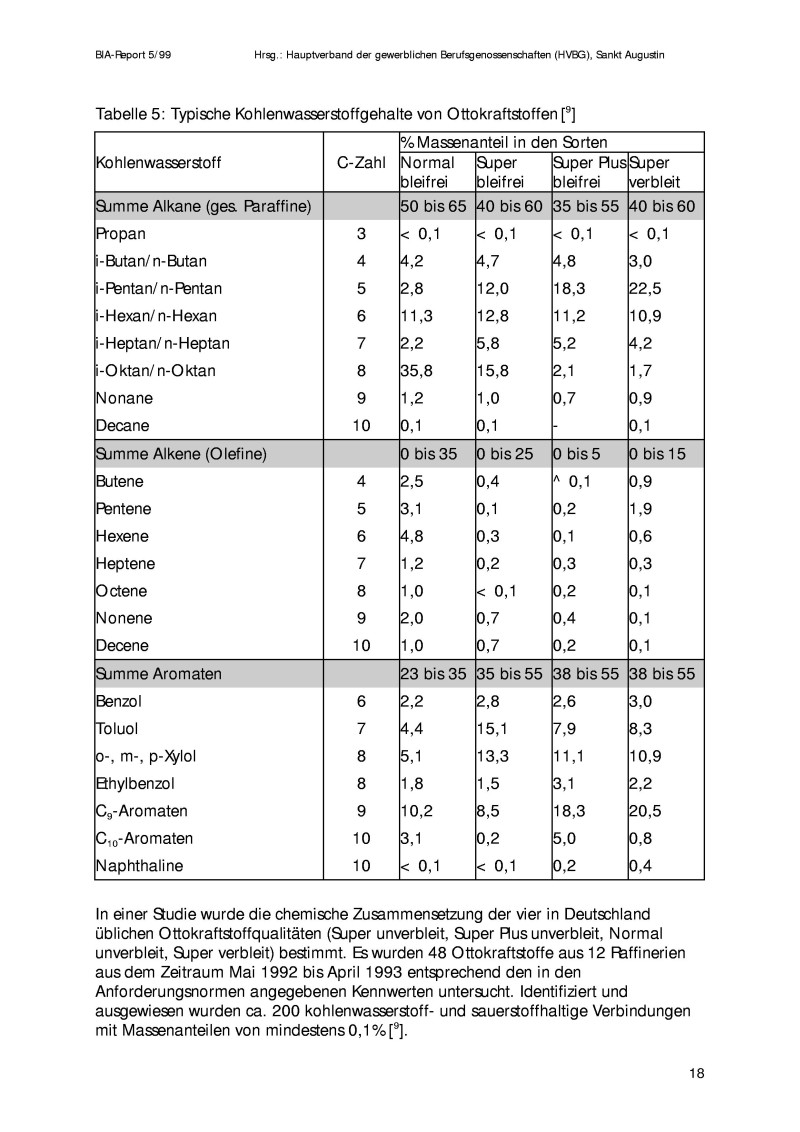
But back on topic: I actually found something what I didn´t had expected: I never knew that my car runs on toluene/xylene. And cumene. These are the
main ingredents of SuperPlus Bleifrei (unleaded) in Europe.
Up to 55% are aromatic compounds, have a look at the attached picture and you´ll see.
benzene: 2,6%
toluene: 7,9%
xylene: 11,1%
C9 aromatics: 18,3% (cumene)
C10 aromatics: 5,0%
So is left the question if these compounds can be isolated and purificated. Only unleaded is worth to think of but also this contains additives - in
special MTBE (methyl-tert. butylester) and methanol/ethanol.
First I will take a look at other fractions of "benzin" - perhaps there is one with even more C9 ? We´ll see.... 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
C6H5-CH(CH3)2, C6H5-C(CH3)=CH2 and C6H5-C(OH)(CH3)2
Organic chemistry is a multiway solution to single problems!
X-C6H5 --> Mg/Ether --> gringnard with CH3-CO-CH3 then H2O/H(+) --> cumol
C6H5-CH3 -KMnO4/H(+)-> C6H5-CO2H -MeOH/H2SO4 -> C6H5-CO2-Me -CH3MgBr then H2O/H(+)-> cumol
C6H5-X + CH3-CHCl-CH3 -Zn dust or Cu dust-> C6H5-C6H5 + (CH3)2CH-CH(CH3)2 + C6H5-CH(CH3)2
All easily separable
C6H5-CH3 + Cl2/hn --> C6H5-CH2-Cl -NaOH-> C6H5-CH2-OH + CH3-CHOH-CH3 -H2SO4 heat-> cumol + other alcohols
C6H5-CO-CH3 -CH3-NO2-> C6H5-C(CH3)=CH-NO2 -Ni/H2-> C6H5-C(CH3)-CH2-NH2 -NaNO2/HCl/H3PO3-> C6H5-CH(CH3)2-CH2OH -H2SO4 reflux hot and then
colder-> cumol
...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
ah, yes.
I had had the hope to aquire some of the compound which is produced in 7 million tons a year, not regulated, not listed and not more dangerous to
health as toluene or xylene.
But for some unknown and secret reason this chemical is not to get, not even at my chem supply - they could order it, but thus takes time and to much
papers are included. And it´s to special - I don´t like to get unnecessary attention. 
But I will for sure not run an Grignard for this, before this is going to happen I go for Fischer Tropsch - this has at least a sporty component. 
Btw. is there a deeper sense in never writing the name of compounds but only line formulas? As in organic chemistry this is quite unusal (and thus a
pain for me). Also I know it as usus to spend some explaining words on the involved reaction, times, temperatures and such. The name of the reaction
would sufficice also.
Look:
Fischer-Tropsch + Haber-Bosch -> any compound you like
Chemistry complete done, lets go have a beer.
Ok. I admit this was exaggerated now. Would you disclose me what Cl/hn is? Unknown to me. The Friedl-Crafts was named by me already. To state it
clearly: All this reactions are messy and not worth the effort. Nothing against you Philou but you know that this is far from reality. I appreciate
the help, realy but somehow the famous reaction comes in mind:
"Black tar from unbelievable expensive and rare compounds"
Sorry for the rant. I had some draw backs today and have to clean the house what isn´t my most beloved work. So i am thin skinned and I don´t know:
are you kidding me?
If somebody finds a solvent/gasoline additive or such with cumene - highly welcome.
I found a nice acetophenone synth from benzoic acid and acetic acid by a tube furnace at not very high temperatures with common catalysts and high
yields. Grignard? No thanks.
Not my day, anyway. 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The 3 last reactions doesn't involve Gringnards.And are pretty easy for lab purpose.
Ar-X + R-X -Zn or Cu dust-> Ar-Ar + R-R + Ar-R
(All easily separable).
C6H5-CH3 + Cl2/hn --> C6H5-CH2-Cl
(Photoactivated halogenation or radical chain to aromatic ring (hn = light couldn't find the greek "nu"
C6H5-CH2-Cl -NaOH-> C6H5-CH2-OH + NaCl
(SN2 of activated benzyl halide by OH(-))
C6H5-CH2-OH + CH3-CHOH-CH3 -H2SO4 heat-> cumol + other alcohols
(Acid catalysed dehydrogenation of alcools to alcene and acid catalysed addition of alcene to alcool)
C6H5-CO-CH3 -CH3-NO2-> C6H5-C(CH3)=CH-NO2
(Condensation reaction of nitromethane with cetocarbon compounds)
C6H5-C(CH3)=CH-NO2 -Ni/H2-> C6H5-C(CH3)-CH2-NH2
(Hydrogenation of alcene and reduction of nitro carbon to amine)
C6H5-C(CH3)-CH2-NH2 -NaNO2/HCl/H3PO3-> C6H5-CH(CH3)2-CH2OH
(Diazotation of primary amine and N2 clivage to get an alcool)
C6H5-CH(CH3)2-CH2OH -H2SO4 reflux hot and then colder-> cumol
(Dehydratation of alcool and rehydratation following markovnikov's rule).
Now if you really want Fisher Tropsch why not Diels Adler condensation of alcynes?
2HC#CH + HC#C-CH(CH3)2 should give you IPB.
There are zillions of other ways, maybe tell me what reactions and conditions you are ready or diposed to perform to get the stuff.
Why do you need those? Phenol and aceton?
You can make them by easier ways than IPB air peroxydation.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
The photocatalyzed looks interesting...
now as I know what this is. 
I admit not all here showed reactions have been known to me and so this is a treasure of wisdom for me anyways. Also as told most is useless for the
told reasons. I just wanted to aquire a legal in masses produced compound...
Phenol and acetone?
Don´t mind getting no answer on this.
What is this for a compound:
C6H5-C(CH3)-CH2-NH2 - NaNO2/HCl/H3PO3
?
If there would be a "+" sometimes it could get to decrypt for me. Just a guess. We have an benzene thread here, where lots of this is
covered btw. You may have a look.
Hey Philou, I admire your knowledge. Sadly I won´t profit much from it as it seems. It is as helpful as looking for cumene in the Beilstein. There
are much more ways! Understand what I want to say? And believe me - there is no offense or else intended by me.
I am only deeply frustrated about us obviously not being able to communicate. I will have a look - perhaps the common level hides somewhere, I will
start looking under my bed.... 
...wasn´t there.... 
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
OK I seem to understand the problem:
I often write:
Ar-R-R'+ R"-X-Y -x-> A-B-C-D
So you often don't see that
-x-> means + x -->
like it is the case in most organic chembooks, the catalyst or ingredients entering the reaction are writen over or inside the arrow.
ex:We should write
C6H6 + HNO3 + H2SO4 + heat --> meta C6H4(NO2)2 + 2H2O + H2SO4
(benzen + nitric acid + sulfuric acid + heat --> metadinitrobenzen)
in practice we write:
C6H6 -HNO3/H2SO4/heat-> mC6H4(NO2)2
(benzen + sulfonitric mix + heat --> metadinitrobenzen)
Much shorter.
So extensivelly explicitated:
Ar-X + R-X + Zn(dust) --> Ar-Ar + R-R + Ar-R + ZnCl2
Ar-X + R-X + Cu(dust) --> Ar-Ar + R-R + Ar-R + CuCl2
(The same reaction is used to copulate 2 chloro 2,4,6 trinitrobenzen ring into hexanitro 2,4,6,2',4',6' biphenyl)
C6H5-CH3 + Cl2 + hv --> C6H5-CH2-Cl + HCl
(toluen + chlorine + UV light --> benzyl chloride + chlorhydric acid)
C6H5-CH2-Cl + NaOH + H2O --> C6H5-CH2-OH + NaCl
(benzyl chloride + aqueous sodium hydroxyde --> benzylic alcohol + sodium chloride)
C6H5-CO-CH3 + CH3-NO2 --> C6H5-C(CH3)=CH-NO2 + H2O
(acetophenone + nitromethane --> 1'methyl,2' nitrostyrene)
C6H5-C(CH3)=CH-NO2 + Ni (Raney) + H2 (1atm 35°C) --> C6H5-CH(CH3)-CH2-NH2
(1'methyl,2' nitrostyrene + Nickel dust Raney catalyst + hydrogen gas at 1 atmosphere and at 35°C --> 2phenylpropan-1amine)
C6H5-CH(CH3)-CH2-NH2 + HNO2 --> C6H5-CH(CH3)-CH2-N#N-OH + H2O
(2phenylpropan-1amine + nitrous acid --> 1hydroxydiazonium,2phenylpropane)
C6H5-CH(CH3)-CH2-N#N-OH + H3PO3 --> C6H5-CH(CH3)-CH2OH + N2
(1hydroxydiazonium,2phenylpropane + phosphorous acid -->2phenylpropan-1ol)
C6H5-CH(CH3)-CH2OH + H2SO4 + 160°C --> C6H5-C(CH3)=CH2 + H2O
(2phenylpropan-1ol + sulfuric acid at 160° --> cumene (2 phenylpropene) + water)
C6H5-C(CH3)=CH2 + H2SO4 + 100°C + H2O -->C6H5-C(OH)(CH3)2
(2phenylpropene + sulfuric acid at 100°C + water --> cumol (2phenylpropan-2 ol)
2HC#CH + HC#C-CH(CH3)2 + pressure --> C6H5-CH(CH3)2
(acetylene + 3 methylbutyne + pressure --> isopropylbenzen)
  
Strange that with such a nickname (Organikum) you don't know all of this... OK I have made 5 mistakes (forgotten two H, put two CH3 too much and
have mistaken myself in the number of carbon a reaction was giving... thus being over the carbon squeletton of cumene)
C6H6 + CH3-CHOH-CH3 --> C6H5-CH(CH3)2 + H2O
Catalysed by H2SO4 and Lewis acids over 160°C.
I have written C6H5-CH2OH instead which of course lead to C6H5-CH2-C(CH3)2-OH.
">Phenol and acetone?
Don´t mind getting no answer on this."
-Strange.Hard to help you if you don't say really what you want to do ... maybe aspirin, vanillin, benzoic acid, phenol, dinitrophenol, some
azodyes, ... that are OTC available could help you by an alternative way.
"What is this for a compound:
C6H5-C(CH3)-CH2-NH2 - NaNO2/HCl/H3PO3?"
Simply 1-amino,2-phenylpropane involved in a diazotation reaction with HONO (nitrous acid) generated in situ (as usual) by NaONO (sodium nitrite) and
HCl (chlorhydric acid).An then the subsequent diazonium hydroxyde upon reducing acidic boiling provides the alcohol (1-hydroxy,2-phenylpropane).
We can communicate!
The sole problem is that I'm rather synthetic and it takes hours to write the way you want me to write...it is rare a chemical name is shorther
than its chemical writing.Except for some useful common products where a natural occuring short name or abreviation emerge (cumene, camphor, TNT,
benzen, tosyl, DDT, ..., salicylic acid, vanillin, ...)
HCl --> hydrogen chloride
C6H5-C(CH3)-CH2-NH2 --> 1-amino,2-phenylpropane
C6H5-CH(CH3)2 --> isopropylbenzen
O2N-CH2-CO-CH2I --> 1-nitro, 3-iodopropanon
O2N-CI2-CI2-NO2 --> 1,2-dinitro, 1,1,2,2 tetraiodoethane
 pretty annoying writing and absolutely heavy and undigestible (maybe you can read
that for hours but in my case, I prefer to write half developped structures... it is more synthetic and visual than IUPAC names. Most chem books
display such writings (only the chem catalogue write the borring text...and as soon as they can use abreviated names or structural drawings). pretty annoying writing and absolutely heavy and undigestible (maybe you can read
that for hours but in my case, I prefer to write half developped structures... it is more synthetic and visual than IUPAC names. Most chem books
display such writings (only the chem catalogue write the borring text...and as soon as they can use abreviated names or structural drawings).
Anyway, we have tried to help you, but in vain   
As we say here:
"We can't help it: if you don't help us, we can't help you!"
"The possible has been done, the impossible is running and for miracles we are working on!"
    
One day we will communicate without troubles; we simply have to make adjustments.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
C6H5-CO2H + CH3-CO2H + heat/catalyst --> C6H5-CO-C6H5 + CH3-CO-CH3 + C6H5-CO-CH3 + H2O + CO2
Thus benzophenone, acetophenone and acetone are produced.
The same reaction can be achieved from drying and then calcinating Calcium benzoate with Calcium acetate.
Ca(O2C-C6H5)2 + Ca(O2C-CH3)2 --> C6H5-CO-C6H5 + CH3-CO-CH3 + C6H5-CO-CH3 + CaCO3
The CaCO3 is recovered and can be recycled with benzoic acid or acetic acid to get Calcium salt.
A tip:
If ever you want an aldehyd...use mixed calcium salts calcination but one of the salts must be Ca(O2CH)2... calcium formate.A slight excess will
favourise aldehyd percentage!
Ca(O2C-R)2 + Ca(O2CH)2 --> R-CO-R + R-CO-H + H-CO-H (or (R)2C=O, R-CH=O and CH2=O (g).
I like those old processes from old books.
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
we can communicate !
It´s possible.
I read patents and chembooks/articles quite often - and thats not the style used there. In internal communications of chemists this will be true and
facile - I am no chemist, sorry - I am much more an engineer.
(Universal genius or universal dillentantism - changes from day to day  ) )
But I won´t bitch - we have arrrived and I am happy about this had happened. I bow my head befor Philou!
It´s a rare gem. Great!
And I see there will be a Name reaction named after Philou Zrealone:
The copulating substituted chlorobenzenes reaction.
(only for students of the higher semesters of course)
  
peace
ORG
[Edited on 4-6-2003 by Organikum]
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
Cumene is found in several gasoline additives which tell to clean the engine and are worldwide available like from SLICK50 and others. (not the PTFE
stuff of course)
(this shows whats the truth behind such promises: the cumene boosts the oktane and is an additive with high energy content. Of course you get the
feeling that your engine runs better so.....)
Thanks to all who helped me here. Also it didn´t bring up this information this thread brought lot´s of knowledge to me.
thx
ORG

[Edited on 13-6-2003 by Organikum]
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Re:Cumene
It 's used also as a pesticide......see,
http://www.chemservice.com/csinewp.htm
greetings org.....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
One gram for 18,40 Dollar ????
Therefore I should get a ten liter bucket with the bucket included!
can´t believe it.
Seven million tons produced per year worldwide .....
|
|
|
leopard
Harmless

Posts: 12
Registered: 14-11-2002
Member Is Offline
Mood: No Mood
|
|
Please forgive my misuse of this forum but I took a look at "todays posts" and saw one about cumene, cumol... and thought, "I
won't look at this one since I know nothing of these compounds", but because i'm so curious I took look anyway. I just want to say it
has been a long time since I have received so much pleasure in reading a thread. It was a delight to see chemistry being used as a universal language
oblivious to language or nationality. I also really appreciated the good spirit in which the exchanges took place, this shows the degree maturity and
respect members poses and brings honour to this forum. Thanks 
|
|
|
solo
International Hazard
    
Posts: 3975
Registered: 9-12-2002
Location: Estados Unidos de La Republica Mexicana
Member Is Offline
Mood: ....getting old and drowning in a sea of knowledge
|
|
Re: Isopropylbenzene
Org. Noted that the price is steep buying it like that but it does give you an idea where it's been used and what typr of products to look for to
get your particular nugget....solo
It's better to die on your feet, than live on your knees....Emiliano Zapata.
|
|
|
thunderfvck
Hazard to Others
  
Posts: 347
Registered: 30-1-2004
Location: noitacoL
Member Is Offline
Mood: No Mood
|
|
Sorry to bring this back from the dead!
But I was looking through my organic chemistry course book, and I saw something that dealt with this very topic!
C6H6 + CH3CH(OH)CH3 --H2SO4/reflux--> C6H5(CH(CH3)2)
I like doing it philou's way 
So yes! They didn't specify anything other than that simple reaction. So, provided you have benzene, isopropyl alcohol, and concentrated H2SO4,
this should be a sinch! I'd try it but I don't have any benzene...
So, Org, give her a shot perhaps?
|
|
|
Organikum
resurrected
    
Posts: 2339
Registered: 12-10-2002
Location: Europe
Member Is Offline
Mood: frustrated
|
|
LOL, thats exactly what I came to myself...
But of course benzene is the problem. Was the problem, as a solution was found.
Soon to come in this theater!
|
|
|
blazter
Hazard to Self
 
Posts: 71
Registered: 3-9-2002
Member Is Offline
Mood: No Mood
|
|
fiberglass industry source
Don't know if it helps any but cumene hydroperoxide (CHP for short) 50% can be bought by the gallon as a non-foaming slow acting catalyst for
fiberglass resins. Knowing the vulnerability of the peroxide bond, it should be easy enugh to break it and get cumene and O2. You'll have to
look for a somewhat serious fiberglass supplier, but this stuff is used all the time in the fabrication business. If you do get the stuff, get some
nitrile gloves, it has a nasty tendency to eat through latex gloves in minutes and burn like a bitch.
|
|
|