| Pages:
1
2
3 |
rot
Hazard to Self
 
Posts: 62
Registered: 26-1-2006
Member Is Offline
Mood: No Mood
|
|
FOX-7
This relatively new explosive has a massive VoD of 8870m/s. not as high as newer explosives like HMX or HNIW, but it's very easy to make once you have
2-methoxy-2-methyl-4,5-imidazolidinedione.
here is the synthesis from megalomania.
The synthesis of 2-methoxy-2-methyl-4,5-imidazolidinedione is here (scroll down).
To make it, the following compounds are needed:
Sodium Methoxide, Acetamidine Hydrochloride and diethyl oxalate.
to make sodium methoxide you can add sodium metal to methanol. pretty simple.'
2CH3OH + 2Na --> 2CH3NaO + H2
For ethyl oxalate you can react ethanol with oxalic acid.
2C2H6O + H2C2O4 --> C6H10O4 + H2O (I think)
For acetemidine hydrochloride I don't know, maybe add hydrochloric acid to acetemide? does anyone have some ideas?
|
|
|
lacrima97
Hazard to Self
 
Posts: 93
Registered: 24-7-2005
Location: MS
Member Is Offline
Mood: experimental
|
|
Man, that sounds like a few expensive/exotic chemicals just to have it decomposed again.
|
|
|
Chris The Great
Hazard to Others
  
Posts: 463
Registered: 29-10-2004
Location: Canada
Member Is Offline
Mood: No Mood
|
|
I have posted a potential synthetic route from FOX-7 to the hypothetical DTTO (detonation pressure calculated to be 5 times greater than that of HMX).
Do a search on DTTO and you should find the diagram of potential reactions.
It might be worth it if you can make DTTO from it, or just as something different. I think other explosives, such as PETN, are much easier to make
for the performance this explosive offers. The only real advantage FOX-7 offers is that it is an insensitive explosive, which the military likes
because their shells and rockets get bumped alot and have to survive flying through walls before exploding.
|
|
|
FrankRizzo
Hazard to Others
  
Posts: 204
Registered: 9-2-2004
Member Is Offline
Mood: No Mood
|
|
BUT...you tie up a great deal of energy in the process, which is later utilized for some very neat applications.
| Quote: | Originally posted by lacrima97
Man, that sounds like a few expensive/exotic chemicals just to have it decomposed again. |
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Megalomania's method of synthesis of Fox-7 actually works. But also it has a trick in one step which is not mentioned directly in his recipe! After
wasting many hours on the process and spending lots of money on expensive chemicals i came into conclusion than the distillation step cannot be
successfully carried out unless, it is done under vaccum. i used a water aspirator for this purpose and it worked! after that my waste of time and
money completely stopped!!
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nitrojet
Megalomania's method of synthesis of Fox-7 actually works. |
Its copied from the attached article, or from where ever these researchers took the process. The article is quite a nice review of FOX-7.
| Quote: | | i came into conclusion than the distillation step cannot be successfully carried out unless, it is done under vaccum |
What distillation step?
[Edited on 25-10-2006 by Axt]
Attachment: FOX-7 a new insensitive explosive.pdf (4.2MB)
This file has been downloaded 4691 times
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
The synthesis method of Fox-7, refers to an intermediate product which is then nitrated and treated with ammonia to give DADNE. It is
2-methoxy-2-methyl-4,5-imidazolidinedione which was the most challanging step towards preparation of FOX-7. A simple distillation procedure is
mentioned for removing the last traces of sodium chloride from our intermediate product:
"At this point a precipitate of sodium chloride should have formed. Pour the contents of the flask over a filter to remove this solid. The salt
collected on the filter paper can be discarded. Pour the liquid filtrate back into the flask and set the flask up for a simple distillation. Very
gently heat the flask to distill off the methyl alcohol. This alcohol should be fairly pure and can be recycled. In this situation the flask is
distilled to dryness to remove all alcohol and leave a solid white powder. When very little alcohol remains reduce the heat to around 30 degrees until
dry"
As a matter of fact such a distillation even if carried out with very gentle heat can have a deteriorationg effect on the highly unstable
2-methoxy-2-methyl-4,5-imidazolidinedione. i tried the procedure may times and i received nothing. however when the process was rendered in a
relatively weak vaccum, i could successfully separate out my product in enough quantities for the next stage which is Nitration and ultimately it gave
afew grams of fox-7.
|
|
|
Axt
National Hazard
   
Posts: 817
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
OK, never clicked the second link. The article I attached just mentions to evaporate at <30°C which makes that step quite trivial, on a small
scale theres no need to complicate things by reclaiming the methanol. Bubble air through it via aquarium pump to aid in evaporation.
[Edited on 25-10-2006 by Axt]
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Yeah, it is a good idea to remove the Methanol by means of a constant stream of air bubbling through it. Water aspirators also can be successfully
used to distill methanol under vaccum. They are simple to set up and are more rapid in removing methanol. at any rate, gentle heating of liquor is not
recommended atall as it cause decomposition of the unstable intermediate.
|
|
|
hanky
Harmless

Posts: 1
Registered: 25-10-2006
Member Is Offline
Mood: No Mood
|
|
This is the informative Link about the FOX-7 where the procedure of its synthesis is given in detail originally
http://www.dsto.defence.gov.au/publications/2412/DSTO-TR-123...
Hope this will improve your sythetic procedures.

Here is the theortical side...or computational side
http://www.dsto.defence.gov.au/publications/2274/DSTO-TR-105...
[Edited on 26-10-2006 by hanky]
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Fox-8
Hypothetically, the two amino groups in Fox-7 molecule can be further oxidized to 1,1,2,2-Tetranitroethylene.
does anybody have any idea of this? Is it possible?
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Possibly, but in the process you might come out with dinitro-diamino-oxirane (OOH!!) or otherwise mess up your fancy double bond.
dinitro-diamino-oxirane
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Nitrojet
Hazard to Self
 
Posts: 56
Registered: 21-10-2006
Member Is Offline
Mood: No Mood
|
|
Yes, The pi bond seems to be the most vulnerable to an oxidative attack, It can be assumaed that any oxidizing agent powerful enough to oxidize Amino
groups, can have its 1st attack to double bond.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Comparative Evaluation of FOX-7
http://www.dtic.mil/ndia/2003insensitive/lochert2.pdf
.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
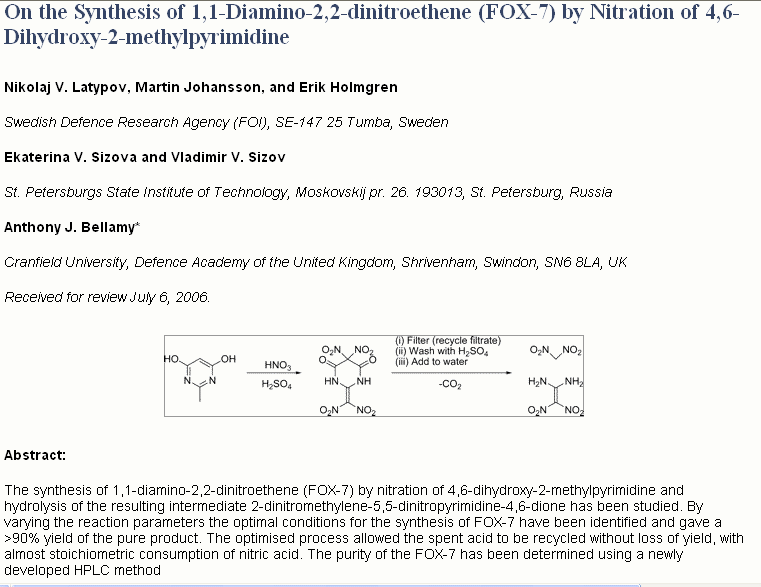
The attached synthesis starting from commercially available 4,6-dihydroxy-2-methylpyrimidine gives a yield reported to be >90%.
Reference: Org. Process Res. Dev., 11 (1), 56 -59, 2007
[Edited on 30-7-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
The byproduct dinitromethane is unstable & decomposes. This patent deals with its synthesis & stable forms for use as a non-Cl-containing
oxidizer: [url][http://www.pat2pdf.org/patents/pat6340780.pdf/url]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Engager
Hazard to Others
  
Posts: 295
Registered: 8-1-2006
Location: Moscow, Russia
Member Is Offline
Mood: Lagrangian
|
|
Dinitrobiurete seems much more easy to make (urea is starting material) and seems identical in power...
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by Engager
Dinitrobiurete seems much more easy to make (urea is starting material) and seems identical in power... |
See the thread New Energetic Materials - Current Research for the structure & synthesis of dinitrobiuret.
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
| Quote: | Originally posted by Engager
Dinitrobiurete seems much more easy to make (urea is starting material) and seems identical in power... |
Assuming one has 4,6-dihydroxy-2-methylpyrimidine (and 100% nitric acid which the synth is easy on, no huge excess needed) the synthesis of fox-7 is
obscenely easy.
Most papers on the synthesis use incomplete reaction times, and longer nitration times and longer crystalization times are necessary.(Can't remember
the paper that said this). Yields reach 90% with longer times. They all state 90% yields, but only one paper has long enough reaction times to allow
this.
EDIT: There is also stability differences, the Dinitrobiurete explodes at 124, and fox7 is classed as an IHE, I don't remember its decomp temp, but it
is high.
[Edited on 2-8-2008 by The_Davster]
|
|
|
Formatik
National Hazard
   
Posts: 927
Registered: 25-3-2008
Member Is Offline
Mood: equilibrium
|
|
| Quote: | Originally posted by Engager
Dinitrobiurete seems much more easy to make (urea is starting material) and seems identical in power... |
That's a good one. VOD estimate is 8.66 mm/ms and P = 33.9 GPa according to this PEP article.
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by The_Davster
Assuming one has 4,6-dihydroxy-2-methylpyrimidine (and 100% nitric acid which the synth is easy on, no huge excess needed) the synthesis of fox-7 is
obscenely easy.
|
Looking at this paten http://www.pat2pdf.org/patents/pat5397781.pdf, this pyrimidine is prepared in Example 1:
| Quote: | Preparation of 4,6-dihydroxy-2-methylpyrimidine
To a solution of sodium ethoxide, prepared from
sodium (36.2 g) and ethanol (1000 ml), diethyl malonate
(114 ml) and acetamidine hydrochloride (71 g) were
added at room temperature, and the mixture was stirred
for 5 hours under reflux. After cooling to room temper-
ature, the resulting solid was collected by fltration and
washed with ethanol and then dissolved in water. The
aqueous solution was acidifed to pH Ca.2 with concen-
trated hydrochloric acid (Ca. 170 ml) under ice-cooling.
The formed precipitate was separated by fltration and
washed with water, ethanol and ether to give 153 g
(81%) of the objective compound.
Melting point: > 300° C.
|
Doesn't sound very difficult, though sourcing or preparing some of the intermediates & reagents might present a challenge.
This compound is available commercially from a number of sources. Finagling a good sized sample might be a possibility.
This company offers it online: http://www.mpbio.com/product_info.php?cPath=491_1_13&pro...
[Edited on 3-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
| Quote: | Originally posted by rot
For acetemidine hydrochloride I don't know, maybe add hydrochloric acid to acetemide? does anyone have some ideas? |
Its preparation is found at Organic Syntheses. It is also used in the synthesis of the alternate starting material
4,6-dihydroxy-2-methylpyrimidine. It is a derivative of acetonitrile: http://www.orgsyn.org/orgsyn/pdfs/CV1P0005.pdf
This company sells it online: http://www.mpbio.com/product_info.php?cPath=491_1_13&pro...
[Edited on 3-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
Ritter
Hazard to Others
  
Posts: 370
Registered: 20-6-2008
Location: Earth
Member Is Offline
Mood: Curious
|
|
Preparation of FOX-7 via nitration of 2-methylimidazole & other heterocycles is given in this patent http://www.pat2pdf.org/patents/pat6312538.pdf. It also contains a lot of chemical & physical data on this explosive.
Also see the prep from 2-methylimidazole in this patent: http://www.pat2pdf.org/patents/pat6113713.pdf
[Edited on 3-8-2008 by Ritter]
Ritter
=============================
\"The production of too many useful things results in too many useless people.\"
Karl Marx
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Ritter, you make some informative posts, but quit double and triple posting, edit your posts to add more info if they are the most recent ones in the
thread.
You may find posts disappearing(read: deleted) if you do not do this
[Edited on 3-8-2008 by The_Davster]
|
|
|
kiko
Harmless

Posts: 6
Registered: 3-11-2010
Member Is Offline
Mood: No Mood
|
|
2-methoxy-2-methyl-4,5-imidazolidinedione
the yield of 2-methoxy-2-methyl-4,5-imidazolidinedione in its preparation is 1 gm !!!!!!!! it must be 37 gm as the paper of FOX-7 A NEW INSENSITIVE
EXPLOSIVE by DSTO and i dont know what is the problem???!!!! any one know the problem please????
|
|
|
| Pages:
1
2
3 |