Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Predicting the color of an organic compound
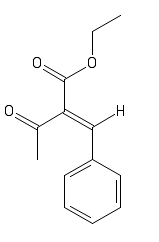
I would like to make some predictions on the color of a compound. I'm at the moment synthesizing para-chlorobenzilidin-bis-acetoacetic ester. I'm
pretty sure the reaction is running slowly but steady. I do see a bright yellow color though. I'm wondering whether it is a byproduct or an
intermediate. I know the stuctures of the intermediates and if possible I would like to predict the color of these but I don't know where to start.
Any ideas?
I'm running 2.05 eq ethyl acetoacetate with p-chlorobenzaldehyde in ethanol with diethylamine as catalyst. It would be nice if the yellow is an
intermediate as that would mean the color can be used to follow the reaction. The color appeared within a couple of hours but doesn't seem to become
stronger. The reaction has been running for 96 hours now, more and more possible product is precipitating out.
Doesn't that look bright yellow to you?
[Edited on 12-3-2019 by Tsjerk]
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
This might be of help: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spe...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4357
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
According to the link provided above, adding an acyl group won't change the wavelength (and thus won't change the colour). Thus, this would be the
same colour as cinnamic acid or (m)ethyl cinnamate (two out of the three I've made as white crystals).
Now, it's possible that there's a very strongly coloured impurity formed along with your product, which is making the solution look yellow. Or that
I'm misunderstanding what the link says.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Based on the color of benzylideneacetone I would be made to believe this compound is yellow. I'll run a search tomorrow and let you know.
I don't think the color of the reaction mixture will fade though but who knows. Usually you don't get visual end points in organic chemistry  . .
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I think the p-chloro is missing from your drawn structure.
If you really want to predict color, a TD-DFT calculation will do it, but those are a bit tricky to run.
Keep in mind the color may be slightly different in ethanol solution vs. the solid state.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Oops, yes. Forgot that one. I will read about TD-DFT.
|
|
|
walruslover69
Hazard to Others
  
Posts: 235
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
you can do a rough back of the envelope particle in a box calculation. Just eye balling it, it looks like the conjugated system might just be large
enough to absorb in the violet.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
The 4-fluoro has been reported as a yellow solid. I would be quite confident in predicting this compound and its 4-chloro derivative are also yellow
solids.
[Edited on 17-3-2019 by Sigmatropic]
|
|
|