r0749547
Harmless

Posts: 14
Registered: 21-12-2018
Member Is Offline
|
|
Relation between solubility and temperature
Is there a real mathematical relation between solubility and temperature?
Exmp. molality ~ epx(temperature),
molarity ~ exp(temperature),
...
I've seen a few documents where they say the relation is exponentially.
But some solubility curves look like they have no relation at all (CaCl2).
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Most things approximately double for every 10oC change,
https://en.wikipedia.org/wiki/Arrhenius_equation
but solubility has to be looked up.
Attachment: AlphabeticalSolubilityOfSalts.ods (55kB)
This file has been downloaded 417 times
[Edited on 11-1-2019 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The relationship between temperature and solubility will vary depending on the solute and solvent in question. Generally, it can be described by the
van't Hoff equation. See: https://en.wikipedia.org/wiki/Van_%27t_Hoff_equation
This equation uses the changes in enthalpy and entropy to describe changes in equilibrium with temperature. Therefore, in order to use it, the changes
in enthalpy and entropy must be known beforehand. Practically speaking, it's often easier to just use a solubility table for the particular substance.
[Edited on 2019-1-10 by Metacelsus]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by Metacelsus  | | This equation uses the changes in enthalpy and entropy to describe changes in equilibrium with temperature. Therefore, in order to use it, the changes
in enthalpy and entropy must be known beforehand. |
You don't need to know the entropy change; just the enthalpy of solution. If you've got that and a solubility at one temp, you can calculate the
solubilities at other ones.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
r0749547
Harmless

Posts: 14
Registered: 21-12-2018
Member Is Offline
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by Metacelsus  | | This equation uses the changes in enthalpy and entropy to describe changes in equilibrium with temperature. Therefore, in order to use it, the changes
in enthalpy and entropy must be known beforehand. |
You don't need to know the entropy change; just the enthalpy of solution. If you've got that and a solubility at one temp, you can calculate the
solubilities at other ones. |
Don't you need the Gibbs free energy? to get the ln(K) = -G0/RT
(I can't write the delta of the Gibbs free energy)
[Edited on 10-1-2019 by r0749547]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
ln(K1/K2)= (-(delta)H/R)*(1/T1-1/T2) The delta H, and the solubility at one temp, is all you need.
This doesn't apply to something like calcium chloride, because you have calcium chloride hexahydrate dissolving at lower temperatures, and then
calcium chloride tetrahydrate dissolving at higher temperatures (and the two hydrates have different enthalpies of solution).
[Edited on 10-1-2019 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
i don't think there is a general formula since every solute and solvent would have different solubility.
edit: changed equation to formula
[Edited on 10-1-2019 by lordcookies24]
|
|
|
r0749547
Harmless

Posts: 14
Registered: 21-12-2018
Member Is Offline
|
|
Can't you use the solubility at two different temps to calculate the change in enthalpy?
[Edited on 11-1-2019 by r0749547]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Quote: Originally posted by r0749547  |
Can't you use the solubility at two different temps to calculate the change in enthalpy?
[Edited on 11-1-2019 by r0749547] |
Yes, that works, too.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
r0749547
Harmless

Posts: 14
Registered: 21-12-2018
Member Is Offline
|
|
Last question.
since solubility tables are always in molality,
to go from the molality to molarity I would need the density of the solution.
But that's hard to find. Do you just assume that the volume stays constant at 100ml?
at first I tought to calculate the mole fraction equilibrium constant and to convert that to the concentration equilibrium constant,
using Kc = Kx(P/RT)^delta(v) (but that only applies to gasses right?)
[Edited on 11-1-2019 by r0749547]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Mole fraction equilibrium constant? I've never heard of that- equilibrium constants are always in molarity for solutes (with gases, you can use Kc or
Kp, but that's not relevant here).
If you ignore the difference between molarity and molality, it really won't make much difference for the van't Hoff eq'n.
For example, let's look at what chemister.ru says about the solubility of benzoic acid in ethanol:
ethanol: 47.1 (15°C) [Ref.]
ethanol: 52.4 (19.2°C) [Ref.]
ethanol: 55.9 (23°C) [Ref.]
Because benzoic acid doesn't dissociate in ethanol, we can just use the solubility as the equilbrium constant (unlike an ionic compound in water,
where we'd have to convert solubility to Ksp). The van't Hoff eq'n gives us 15.2 kJ/mol for the enthalpy of solution (based on the 15 and 23 oC
values).
Using this enthalpy, we can find the solubility at 19.2 oC as 51.6 g/ 100 g solvent, which is pretty close.
[Edited on 11-1-2019 by DraconicAcid]
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
r0749547
Harmless

Posts: 14
Registered: 21-12-2018
Member Is Offline
|
|
Quote: Originally posted by DraconicAcid  | Mole fraction equilibrium constant? I've never heard of that- equilibrium constants are always in molarity for solutes (with gases, you can use Kc or
Kp, but that's not relevant here).
If you ignore the difference between molarity and molality, it really won't make much difference for the van't Hoff eq'n.
For example, let's look at what chemister.ru says about the solubility of benzoic acid in ethanol:
ethanol: 47.1 (15°C) [Ref.]
ethanol: 52.4 (19.2°C) [Ref.]
ethanol: 55.9 (23°C) [Ref.]
Because benzoic acid doesn't dissociate in ethanol, we can just use the solubility as the equilbrium constant (unlike an ionic compound in water,
where we'd have to convert solubility to Ksp). The van't Hoff eq'n gives us 15.2 kJ/mol for the enthalpy of solution (based on the 15 and 23 oC
values).
Using this enthalpy, we can find the solubility at 19.2 oC as 51.6 g/ 100 g solvent, which is pretty close.
[Edited on 11-1-2019 by DraconicAcid] |
But what do you do if it does dissociate and you do need the molarity for the Kst?
Do you simply assume the 100g of water to be 100ml and neglect the volume change of mixing? (ex. NaCl in water)
And about the mole fraction equilibrium constant...
It stands in one of my books I need to know for my exams 
[Edited on 11-1-2019 by r0749547]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4355
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
If you need the molarity for a dilute solution, then just assume that the 100 g of water makes 100 mL of solution. If it's a concentrated solution
(such as saturated sodium chloride in water), then it's not going to work anyway because the ionic strength mucks things up, and you have to use
activities instead of concentrations. Even without considering ions, if you have a solution that is 10% non-ionizing solute (such as 2-butanone), the
solution doesn't act as a pure aqueous solution (it's only 90% water). That will change K for anything dissolving in it- ionic compounds will be less
soluble, and organic compounds will be more soluble.
Even then, the van't Hoff eq'n isn't exact, because the enthalpy of solution will vary slightly with temperature.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Morgan
International Hazard
    
Posts: 1705
Registered: 28-12-2010
Member Is Offline
Mood: No Mood
|
|
Is there a solid substance that is more soluble at a lower temperature than at a higher temperature?
[Edited on 12-1-2019 by Morgan]
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Many lantjanide series salts do this. Ce2(SO4)3 is one if I am not mistaken.
For something a bit less exotic, calcium carbonate. Tdep did a nice video on this on his extractions and ire channel.
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
the van 't Hoff equation seems to be applicable only over a range of temperature that needs to be chosen by refering to a solubility table or chart.
No doubt useful for optimising/fine-tuning an industrial application but not much use to me.
Praseodymium sulfate* has a third-order behaviour (see file I posted above)
- fit that with a van 't Hoff equation 
* the first one I spotted, many other salts have anomalies
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
@Sulaiman I can't open the ods file with excel, something unreadable
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
The file is just a version of the Wikipedia page https://en.wikipedia.org/wiki/Solubility_table
Attachment: AlphabeticalSolubilityOfSalts.xls (401kB)
This file has been downloaded 422 times
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Quote: Originally posted by j_sum1  | | For something a bit less exotic, calcium carbonate. Tdep did a nice video on this on his extractions and ire channel. |
Really? Did he? I can't find that.
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Quote: Originally posted by DraconicAcid  | Quote: Originally posted by Metacelsus  | | This equation uses the changes in enthalpy and entropy to describe changes in equilibrium with temperature. Therefore, in order to use it, the changes
in enthalpy and entropy must be known beforehand. |
You don't need to know the entropy change; just the enthalpy of solution. If you've got that and a solubility at one temp, you can calculate the
solubilities at other ones. |
Yes. Knowing the enthalpy and the solubility at one temperature is equivalent to knowing both the enthalpy and entropy.
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Some reagents do follow a simple solubility curve if you plot solubility agaist temperature. There is always a caveat. Reagents which react with the
solvent do not, even if it is only hydrate formation. The curve is usually linear or exponential and can be used as a guide for recrystallising
reagents. I have attached 2 curves BaNO3 which is just about linear and CuSO4 which approximates to exponential.
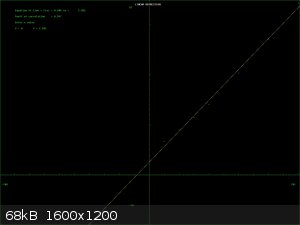 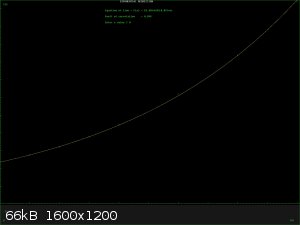
If you're not part of the solution, you're part of the precipitate.
|
|
|
Quibbler
Hazard to Self
 
Posts: 65
Registered: 15-7-2005
Location: Trinidad and Tobago
Member Is Offline
Mood: Deflagrated
|
|
If you assume that the solute is forming an "ideal solution" then the mole fraction of solute (x) at temperature T is related to the fusion enthalpy
(effectively going into solution is similar in energy to melting)
ln(x)=(/\H(fusion)/R)*(1/T(fusion)-1/T)
|
|
|