Outback1850
Harmless

Posts: 2
Registered: 11-12-2018
Member Is Offline
|
|
Alright looking for insight
Im quite comfortable manipulating oubjects in 3d space. I would like to know how expirenced seasoned chemists actually work, take benzene for example
a planer eqilateral hexagon sp2 hybridized now all the orbitals are superimposed and this is where i get into trouble I imagine this but is this
actually a worth wild and consctructive way to think any responce would be appreciated im just trying to develope an intuitive sence for things thanks
in advance
|
|
|
j_sum1
Administrator
       
Posts: 6336
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Not sure what your question is. Nor why two of your cabons have 5 electron clouds.
This stuff is well understood and fully described in good organic chem textbooks. You might look in the forum library.
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
I think the "5" electron clouds are supposed to be three sp2 hybridised orbitals, and one pure p orbital perpendicular to the
sp2 orbitals.
|
|
|
stamasd
Hazard to Others
  
Posts: 137
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I tend to mentally represent the benzene structure as a ring covered on both sides by fuzzy discs. The ring is the carbon backbone, and the fuzzy
discs the shared electrons.
|
|
|
Sulaiman
International Hazard
    
Posts: 3727
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
some nice explanations and diagrams
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Che
mical_Bonding/Fundamentals_of_Chemical_Bonding/Electrostatic_Potential_maps
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
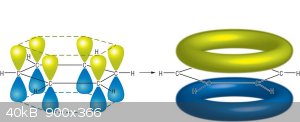
From what I understand the shared orbitals are fully delocalized and truly form two (here blue and yellow) donuts.
|
|
|
Outback1850
Harmless

Posts: 2
Registered: 11-12-2018
Member Is Offline
|
|
Thanks for the quick replys. My question is, what should i imagine when thinking of molecules; spacefilling diagrams, molecular orbitals or does a
pragmatist despence with this sort of thing and go with a more 2dimensional style?
|
|
|
j_sum1
Administrator
       
Posts: 6336
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
You think of an aromatic ring as a planar, remarkably stable structure.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Outback1850  | | Thanks for the quick replys. My question is, what should i imagine when thinking of molecules; spacefilling diagrams, molecular orbitals or does a
pragmatist despence with this sort of thing and go with a more 2dimensional style? |
As a pragmatist, I would say the 2D model is fine most of the time, as long as you remember a benzene ring doesn't have three double and three single
bonds, but more a donut as in the figure. Or you could even see them separately as when drawing reaction mechanisms involving these electrons. For
example :
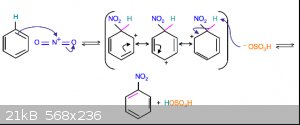
[Edited on 24-12-2018 by Tsjerk]
|
|
|
andy1988
Hazard to Others
  
Posts: 135
Registered: 11-2-2018
Location: NW Americus ([i]in re[/i] Amerigo Vespucci)
Member Is Offline
Mood: No Mood
|
|
This 2D/3D model talk reminds me of an interesting article I read a while ago:
| Quote: | "Chemists have long had to choose between these two stalwart reaction classes, both presenting marked advantages, but also shortcomings," say Baran,
holder of the Darlene Shiley Chair in Chemistry at Scripps Research. "Combining these two reactions solves this dichotomy by leveraging the strengths
of both to provide a reliable and versatile strategy for producing complex molecules."
C-C cross coupling, a bond-forming method that is highly reliable and controllable, has long been the method of choice in the pharmaceutical industry
for synthesizing the skeletons of drug candidates. However, the method is limited in its ability to construct complex three-dimensional architectures,
resulting in a disproportionate number of flat drug molecules—a characteristic that potentially presents a hurdle to creating new drugs for
increasingly difficult biological targets. Cycloaddition reactions, in contrast, offer the ability to build highly complex 3-D shapes in a single
step, but different types cycloaddition reactions required highly customized preparation, which limited their utility. [1]
[Edited on 24-12-2018 by andy1988] |
|
|
|
stamasd
Hazard to Others
  
Posts: 137
Registered: 24-5-2018
Location: in the crosshairs
Member Is Offline
Mood: moody
|
|
I prefer the benzene ring representation without double links:

|
|
|
Dr.Bob
International Hazard
    
Posts: 2757
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
In simple benzene, I do think of the carbons as all equivalent, and the electrons as a donut on both sides of the ring. To really understand
chemistry, you then need to think of each carbon based on the electron density on it. So for carbon with a "H" on it, there is an "average" amount
of electron density on the carbon. If the carbon has an alkyl chain or methoxy, that will donate more electrons to the carbon, so the donut is
thicker on that carbon. If the carbon has a nitro on it, or another element that is more electronegative than carbon, it will suck electrons away
from it, and the carbon will have a small donut of electrons near it. So that controls which carbons will react with various other reagents.
No matter what reactions you memorize, the key to organic chemistry is to remember that like charges repel and opposites attract. That, and sterics
control most reactivity and where molecules react. And I always liken bulky sterics to fat people trying to procreate. The bigger you are, the
less easy it is to do that. A tert-butyl group works the same way, crowding out any courting reagents from coming close to the carbon it is on.
|
|
|
CharlieA
National Hazard
   
Posts: 646
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
I always said that sex could be an analogy for anything and everything!
Merry Christmas
Charlie
|
|
|