Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Resazurin synthesis (planning first steps)
I'm wondering if the best starting material for 4 nitro resourcinol is p-amino phenol followed by sulfonation then fusion with hydroxide and oxidation
of the amine. Or resorcinol followed by nitration. Or something else entirely?
Resourcinol is needed again later. But has entirely the wrong directing potential for the target intermediate.
P-amino phenol is easy to get hold of but would the amine survive or need protection? Or change the order of steps?
The last step with MnO2 seems easy enough once the two rings are joined.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
Resazurin is a trivial name which tells us nothing about its constitution. In order to answer your question we need to know what the molecule looks
like.
However, preparing 4-nitroresorcinol from p-aminophenol looks like a hard task though mononitration of resorcinol is tricky too but its not the
4-nitro compound you need, if I recall correctly, its the 4-nitroso-resorcinol. Again the problem may be controlling the level of substitution to the
monosubstituted resorcinol.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
you can directly nitrosate resorcinol to 4-nitrosoresorcinol -https://onlinelibrary.wiley.com/doi/10.1002/cber.19020350463
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Quote: Originally posted by Swinfi2  | I'm wondering if the best starting material for 4 nitro resourcinol is p-amino phenol followed by sulfonation then fusion with hydroxide and oxidation
of the amine. Or resorcinol followed by nitration. Or something else entirely?
Resourcinol is needed again later. But has entirely the wrong directing potential for the target intermediate.
P-amino phenol is easy to get hold of but would the amine survive or need protection? Or change the order of steps?
The last step with MnO2 seems easy enough once the two rings are joined. |
How did you come up with nitro resorcinol? Do you have a pathway in mind?
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
I mistakenly put nitration (and 4-nitro...) when i meant nitrosylation and 4-nitroso...
The TM looks like this:
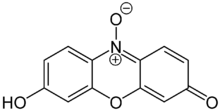
I'll try the route Cureus suggested first (once I get the materials).
[Edited on 27-11-2018 by Swinfi2]
[Edited on 27-11-2018 by Swinfi2]
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Ok, and then what? Do you have a plan? How did you come up with nitrosoresorcinol? It didn't fall out of thin air I guess.
|
|
|
Boffis
International Hazard
    
Posts: 1879
Registered: 1-5-2011
Member Is Offline
Mood: No Mood
|
|
I wonder if you could do this as a one-pot synthesis by dissolving 2 mol-equiv of resorcinol on ice cold conc. sulphuric acid, adding 1 mol-equiv of
sodium nitrite, heat to complete the condensation and ring closure then add 1-mol eqiv o MnO2 to oxidize the tertiary amine?
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Even if it succeeds, you would still need to take exquisite care to avoid reduction to resorufin.
I've found, while working with the compound (Sigma), that it is surprisingly easy to accidentally do this. Low pH seems to be a factor (I was trying
to intercalate it into an uncured polyester with a lot of free monomer present).
Resazurin in most commonly used as an indicator of metabolism--it isn't strongly fluorescent, but once reduced, is bright pink (UV) with a high
quantum yield. Quite cool, actually.
O3
See: https://en.wikipedia.org/wiki/Resazurin
[Edited on 30-11-2018 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
I was wondering myself if a 1-pot might be viable, after seeing chemplayers video(s) on nitrosation they look to be relatively straightforward.
I think I'll try this small scale. See if it works and troubleshoot if not.
My plan is to use an ice/salt bath aiming for about -10-20c, I'll need to rig up some mag stirring too.
Add in order keeping temp low as possible and pre-chilling where possible;
2 eq resorcinol in acetone:water 80:20 (as per wiki's linked patent)
1 eq sodium nitrite
2 eq 50% sulphuric acid (diluted to reduce exotherm)
At this point there should be a blue colour and NOx offgas.
Wait til the blue nitrous acid has mostly gone
1 eq MnO2
Warm to rt leave to react. (3hrs?)
Bicarb to neutralise.
My intended use for it is as a specific pH indicator.
Just need to work out how best to purify out the resazurin from a complex mixture. And thanks for the advice about avoiding potential reduction. 
I'll video it when I've got everything together.
|
|
|