Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Analysis of common OTC and homemade solvents by proton NMR
I finally got around to doing something I've been meaning to do for a while- taking samples of several OTC and homemade solvents from my home lab in
to my university and seeing how pure they are by proton NMR. These are my results.
All samples were run on a Bruker 400 MHz Avance III NMR spectrometer.
Toluene:
"KleanStrip" brand toluene, over two years old. The sample was taken directly from the original can and diluted with deuterated chloroform. I promise
there is a solvent peak on there, it's just really small, because the sample was very concentrated.

The aromatic peaks and the methyl peak of toluene show in the expected locations and ratio. A small water peak can be seen as well. When this is
integrated and compared to the toluene peaks, it appears to indicate that the toluene contains approximately 1% water. Otherwise, it looks to be quite
clean.
Acetone:
"Crown" brand acetone, purchased a few months ago. The sample was taken directly from the original can and diluted with deuterated chloroform.

This one turned out beautifully: acetone's singlet is the only significant peak. I'm very surprised that there is no water visible.
Diethyl ether:
Fractionally distilled from "Johnsen's" brand starting fluid, which also contains heptanes and some lubricant. A 400 mm Vigreux column was used in the
distillation. The ether has been stored in a glass bottle in my freezer since then. The sample was taken from the bottle and diluted with deuterated
chloroform.
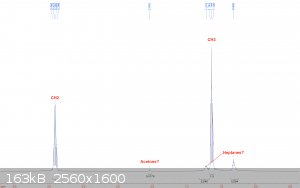
Diethyl ether's CH2 and CH3 peaks show up right where they're supposed to be, and in a perfect 2:3 ratio for integrals. A peak
that could be acetone is visible, but I think this is most likely because I prepared this sample right after the acetone one, and though I used a new
pipette, I reused the same bulb, so I think it is merely contamination of the sample. The more troublesome peaks are the spurious ones in the
aliphatic region. These are likely due to heptanes that distilled over with the ether. Fortunately, heptanes should not interfere in any reactions
that ether would be used as a solvent for. More importantly though, there is no water visible, so drying it for Grignards etc should be relatively
easy.
Benzene:
Home made, by decarboxylation of sodium benzoate (food grade from eBay), followed by a second simple distillation. This prep was carried out almost
two years ago, and the benzene has been stored in a glass bottle at ambient temperature since then. The sample was taken from the bottle and diluted
with deuterated chloroform.


For this one I ran a C13 NMR as well, because I saw that on the proton spectrum, although it first appeared to be a single peak as expected, it is
actually two very close peaks of different heights. I wasn't sure whether this was just some kind of weird issue with the machine, or if it was an
impurity. I'm leaning towards an issue with the machine, because the C13 spectrum looks very clean. A small water peak is visible in the proton
spectrum, which integrates to about 1%. Honestly this one looks much better than I had expected it to, considering the nasty orange goop that it was distilled from!
Dichloromethane:
Distilled from "KleanStrip" brand sprayable paint stripper, followed by repeated water washes to remove methanol, drying over calcium chloride, and
redistilling. This purification was carried out about a year ago, and the DCM has been stored in a glass bottle in my freezer since then. The sample
was taken from the bottle and diluted with deuterated acetone. In retrospect, I'm not sure why I didn't just use chloroform, since their signals
really aren't that close.

It appears to be pretty pure. I'm not sure what the small spurious peaks on either side of the DCM peak are, or if they are indeed anything at all.
There is a very small methanol signature, though it is not visible in the posted picture. It integrates for <0.1%. I'm impressed that the
separation from methanol was this efficient.
Chloroform:
Home made using the haloform reaction over a year ago. It has been stored unstabilized at ambient temperature since then in an amber glass bottle. The
sample was taken from the bottle and diluted with deuterated methanol.
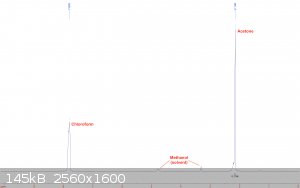
This one was rather disappointing. A large acetone peak is visible, which comes out to about 13% by integration when the 6:1 proton ratio is accounted
for. Washing the chloroform a few times with water, drying, and redistilling should probably remove the majority of the acetone.
Overall, I'm quite impressed by the relative purity of these solvents. With the exception of the chloroform, these all seem perfectly suitable for
most reactions. I attached a good reference for NMR solvent shifts. I've also included a compressed folder containing all of the unprocessed spectra
in case anyone else with NMR software would like to take a closer look.
Attachment: NMR Chemical Shifts of Trace Impurities.pdf (661kB)
This file has been downloaded 415 times
Attachment: Solvents NMR Data.zip (3.5MB)
This file has been downloaded 409 times
|
|
|
UC235
National Hazard
   
Posts: 565
Registered: 28-12-2014
Member Is Offline
Mood: No Mood
|
|
Very interesting. Was the chloroform washed or distilled in any way or just collected from the bottom of the reaction mix? I'd be unsurprised to see
so much acetone in a crude product, especially if the bleach wasn't used in particularly large excess. That is somewhat troubling if this is
post-drying and distillation though. Acetone forms a high-boiling azeotrope with chloroform that might complicate its removal.
Sulfuric acid is always an option.
|
|
|
morganbw
National Hazard
   
Posts: 561
Registered: 23-11-2014
Member Is Offline
Mood: No Mood
|
|
Good info.
Thanks
|
|
|
Texium
Administrator
       
Posts: 4580
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Quote: Originally posted by UC235  | | Very interesting. Was the chloroform washed or distilled in any way or just collected from the bottom of the reaction mix? I'd be unsurprised to see
so much acetone in a crude product, especially if the bleach wasn't used in particularly large excess. That is somewhat troubling if this is
post-drying and distillation though. |
It was post-drying over calcium chloride and distillation. I'm
surprised too. I had assumed that most of the unreacted acetone would have gone into the aqueous phase, but I suppose it could have been salty enough
to prevent that, and some washes with pure water prior to drying and distillation would be more effective at removing it.
I made the chloroform a long time ago though, and can't recall the exact ratios of hypochlorite and acetone used. It would have been made with 10%
NaOCl pool chlorinator as the hypochlorite source, and the same brand of acetone that I analyzed above.
|
|
|
monolithic
Hazard to Others
  
Posts: 436
Registered: 5-3-2018
Member Is Offline
Mood: No Mood
|
|
Thank you for taking the time to write this, very useful to know. A few years ago I managed to find a website with a small database of purity (based
on NMR samples) for common OTC solvents. I think it was related to a fire department -- I can't remember. Either way, I lost the link. 
Edit: Found it! Choose from the drop-down menu at the top of the page: http://ilrc.ucf.edu/sample_detail.php?sample_id=2
[Edited on 18-11-2018 by monolithic]
|
|
|
Mr. Rogers
Hazard to Others
  
Posts: 184
Registered: 30-10-2017
Location: Ammonia Avenue
Member Is Offline
Mood: No Mood
|
|
This is wonderful. The acetone doesn't surprise me and I'd bet most of the store brands would appear similar.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
I must say that I'm surprised there is no trace of denatonium benzoate in the acetone.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
Mr. Rogers
Hazard to Others
  
Posts: 184
Registered: 30-10-2017
Location: Ammonia Avenue
Member Is Offline
Mood: No Mood
|
|
I've only ever seen this in acetone marketed for nail polish remover - at least they seem to be the only acetone that lists denatonium benzoate on the
label.
The HW store acetone usually just lists acetone, in contrast to the other various solvents that are a hydrocarbon witches brew.
[Edited on 18-11-2018 by Mr. Rogers]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Would a trace of denatonium benzoate show up? The concentration is surely very low.
|
|
|
Mr. Rogers
Hazard to Others
  
Posts: 184
Registered: 30-10-2017
Location: Ammonia Avenue
Member Is Offline
Mood: No Mood
|
|
It's usually something like %0.0006 denatonium benzoate in SDA 40B ethanol.
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
well the benzene looks cool, very pure indeed. orange goop you lost.
i will try making benzene from PET, maybe my professor will let me analize it at university
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
12thealchemist
Hazard to Others
  
Posts: 181
Registered: 1-1-2014
Location: The Isle of Albion
Member Is Offline
Mood: Rare and Earthy
|
|
Fascinating and reassuring results! Many thanks for taking the time to carry these out; it's worth knowing roughly how pure one's solvents are. I may
be from across the pond, but still very useful information.
|
|
|
Tsjerk
International Hazard
    
Posts: 3032
Registered: 20-4-2005
Location: Netherlands
Member Is Offline
Mood: Mood
|
|
Very nice, it confirms acetone is dry of the shelf.
|
|
|
chloric1
International Hazard
    
Posts: 1140
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Fascinating thread! Good job! Wonder if I could get someone to run an NMR for me if I require.
Fellow molecular manipulator
|
|
|