benzophenone
Harmless

Posts: 2
Registered: 7-11-2018
Member Is Offline
|
|
Tricyclopentyl alcohol treated with HCL
Hello. I have been having a lot of trouble with the reaction shown in this image.
I just can't picture how it would lead to a tetra-substituted alkene with formula C17H28. Can anyone come up with a representation
of the product?
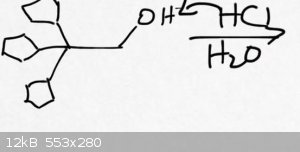
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
The first thing that's going to happen is that you'll get rid of the hydroxyl to form a primary carbocation. Primary carbocations are unstable as all
get out, so you'll immediately have a rearrangement where one of the cyclopentyls shifts to that primary carbon.
That gives you a tertiary carbocation. Remove an H+ from one of the carbons next to it, and viola! An alkene! If you take off the right one, it will
be tetrasubstituted.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
benzophenone
Harmless

Posts: 2
Registered: 7-11-2018
Member Is Offline
|
|
Thank you so much. You have made a perfect explanation. Following your instructions, I would end with this molecule, correct?
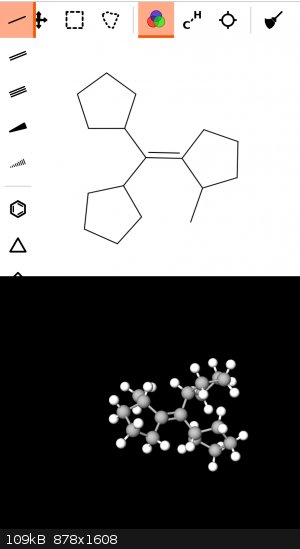
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
How would it dehydrate when the solvent is H2O?
|
|
|
Loptr
International Hazard
    
Posts: 1348
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by DraconicAcid  | The first thing that's going to happen is that you'll get rid of the hydroxyl to form a primary carbocation. Primary carbocations are unstable as all
get out, so you'll immediately have a rearrangement where one of the cyclopentyls shifts to that primary carbon.
That gives you a tertiary carbocation. Remove an H+ from one of the carbons next to it, and viola! An alkene! If you take off the right one, it will
be tetrasubstituted. |
Wouldn't you also get substitution, albeit to a lesser extent? Or is the rearrangement too fast for that to happen? If a more stable
secondary/tertiary carbocation can be formed, will it be formed compared to other possibilities?
| Quote: | | Other common strong acids such as HCl, HBr or HI are less suitable catalysts as nucleophilic substitution reactions will probably interfere
|
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch15/ch15-4...
EDIT: Ah, I also see that no deprotonation can occur due to that being a quaterniary carbon, so the alkyl shift occurs instead.
Another question, how do you get the removal of the hydrogen to an alkene? The carbocation has been resolved at this point, so you just have an
unsymmetrical alkane.
EDIT: Also, with the water present, wouldn't this reaction take the more favorable E2 route, rather than an E1 (I supposed you meant this because of
the immediate carbocation formation)? Water acting as the base? With the E2 you wouldn't get the intermediate carbocation. Unless because of that
carbon already being quaterniary, a carbocation is formed?
[Edited on 7-11-2018 by Loptr]
"Question everything generally thought to be obvious." - Dieter Rams
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by benzophenone  | | Thank you so much. You have made a perfect explanation. Following your instructions, I would end with this molecule, correct?
|
you would get this.This is the reaction that is happening -https://en.wikipedia.org/wiki/Wagner%E2%80%93Meerwein_rearra...
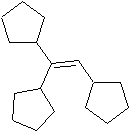
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
OP said tetrasubstituted alkene, that one is trisubstituted.
This would be the correct tetrasubstituted product:
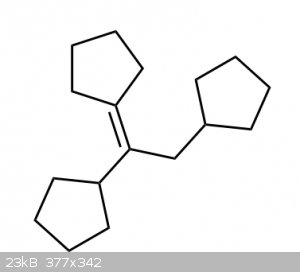
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Ya I know that.I thought the OP made a mistake
in the structure
| Quote: | | This would be the correct tetrasubstituted product |
but there would be a lot of torsional strain in that
molecule.It doesn't look like it would form naturally or freely.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4333
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
| Quote: |
Wouldn't you also get substitution, albeit to a lesser extent? Or is the rearrangement too fast for that to happen? If a more stable
secondary/tertiary carbocation can be formed, will it be formed compared to other possibilities? |
As I understand it, the primary carbocation is so unstable that rearrangement is virtually simultaneous with ionization.
| Quote: | Another question, how do you get the removal of the hydrogen to an alkene? The carbocation has been resolved at this point, so you just have an
unsymmetrical alkane.
EDIT: Also, with the water present, wouldn't this reaction take the more favorable E2 route, rather than an E1 (I supposed you meant this because of
the immediate carbocation formation)? Water acting as the base? With the E2 you wouldn't get the intermediate carbocation. Unless because of that
carbon already being quaterniary, a carbocation is formed? |
After rearrangment, you still have a carbocation, and water will deprotonate it to give an alkene. It's not going to be E2, because water's not a
strong base, and you can't get the rearrangement without forming a carbocation.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|