sumatra
Harmless

Posts: 25
Registered: 15-8-2018
Member Is Offline
|
|
"Broken" thermometre
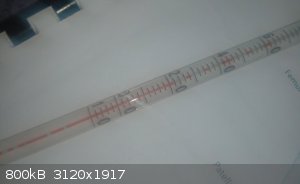
So I had a lab incident recently, I didn't have any mineral oil on hand so I used cooking oil for an oil bath. However it caught fire, I managed to
get everything under control and after I cleaned my glassware I noticed that my alcohol thermometre (-10 C/200 C) has little gaps in the alcohol
column (see image below). It does not appear to be broken anywhere and upon heating and cooling the gaps do not dissapear. I haven't tried heating
untill everythimg reaches the bulb at the top yet.
Is this fixable and will the thermometre be accurate afterwards?
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
I'd try cooling until everything is in the bulb at the bottom before I tried heating it.
It might be OK afterwards- the only way to know is to test it.
Testing in ice, and in boiling water will give you a pretty good idea if it is right.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Sometimes those gaps can be gotten rid of by condensing everything into the bulb with dry ice, or this and a combination of careful warming on the
rest of the thermometer. It's also very easy to break a thermometer attempting this so proceed at your own risk.
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
I'd say gently tapping might release it - but I don't want to risk breakage. What about vigorous shaking? Will that dislodge it?
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
Sulaiman
International Hazard
    
Posts: 3724
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
My score so far: two fixed and one exploded.
The length of stem between 0 and the bulb indicates that a very low temperature will be required to force all of the liquid into the bulb,
maybe even colder than dry ice.
I did not have a low enough temperature source to try with mine.
I have 'repaired' a mercury thermometer by CAREFULLY heating the bulb until the gap entered the small bubble at the end of the stem.
The liquid then forms a continuous thread on cooling ... one fixed thermometer 
This is also how I 'exploded' a different mercury thermometer 
The thermometer in the photograph has so many breaks in the thread that I suspect that neither cooling nor heating will easily fix it.
I agree with DrP that tapping may work,
I'd hold the thermometer vertically above a piece of wood (or hard plastic) and release it and catch it after the bounce,
start about 1cm above the wood and repeat and increase height until the thermometer is either repaired or broken 
I think that the bulb should hit the wood with the stem as vertical as practical for even stress on the bulb..
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
Quote: Originally posted by Sulaiman  |
I'd hold the thermometer vertically above a piece of wood (or hard plastic) and release it and catch it after the bounce,
start about 1cm above the wood and repeat and increase height until the thermometer is either repaired or broken 
I think that the bulb should hit the wood with the stem as vertical as practical for even stress on the bulb..
|
I re-agree - this is what I'd try - as long as you are aware that breakage is a real and likely event.... I'll guess the results will be similar to
what you have tried already with 2 fixes to every 1 breakage or so. Good luck! 
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Instead of heating the bulb to make the alcohol rise to the top, couldn't you heat the upper part of the thermometer to vaporize that little section
of alcohol?
I guess this will increase the pressure inside and might cause breakage. You probably should not overheat. You don't want to reach the boiling point
of the alcohol.
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
How about centrifuging?
I'd try putting it in a (plastic) test tube attached to a rope and swing it around.
If you can generate enough g-force, it might pull the alcohol down towards the bulb.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Quote: Originally posted by phlogiston  | How about centrifuging?
I'd try putting it in a (plastic) test tube attached to a rope and swing it around.
If you can generate enough g-force, it might pull the alcohol down towards the bulb.
|
The alcohol expands due to thermal expansion, making it to contract centrifuging it wouldn't work. Try compressing water by just spinning it... Same
idea
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Metacelsus
International Hazard
    
Posts: 2539
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
I think Phlostigon intends for centrifugation to move the separated part down towards the rest of the alcohol, not actually compress the alcohol. This
might work but I would be careful since thermometers are fragile.
|
|
|
phlogiston
International Hazard
    
Posts: 1379
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Yes, exactly.
And now that we talk about this, it seems to me that the -gas bubbles- will be able to compress a little due to the increased force of the alcohol
above them. If they get small enough, that may also aid in allowing the separated alcohol to get past the air bubbles and join the larger mass.
As with all the above methods, you risk cracking it.
Perhaps supporting the thermometer in a tube filled with sand as you centrifuge it may help it survive.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
j_sum1
Administrator
       
Posts: 6335
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Put the end in a hot flame and seal the glass.
It is always useful to have another stirring rod. 
|
|
|
Ubya
International Hazard
    
Posts: 1247
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
maybe this only if it breaks
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|