| Pages:
1
2
3
4
..
14 |
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Here's the barium acetate solution that was left behind after gravity filtering. It has a slight sulfurous odor, and it is obviously impure. I'm not
exactly sure what the brown color is... I believe that iron (iii) acetate is rather insoluble. There were iron filings in the barium carbonate; they
stuck to the stirbar.
My plan is to add some sodium hydroxide solution, which should precipitate barium hydroxide octahydrate. Heating to boiling and doing a hot filtration
will hopefully get rid of any carbonates. I'll probably recrystallize the product a few times for purity then dry it to the monohydrate in a warm oven
(easier than using a desiccator).

I have some disgusting and toxic barium waste sludge that I have to dispose of. I'm pretty sure I should not put it down the drain. Right now it is
just sitting in a container while I figure out what to do with it.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Today I prepared ethyl benzoate from benzoic acid, dry ethanol and sulfuric acid on 0.5 molar scale. For some reason the reaction boiled out of
control, flooded the condenser and made a wintergreen-smelling volcano inside my lab. Of course this happened when I left the reaction unmonitored for
a few minutes. Only a small portion of the reaction mixture was lost though, and I ended up with 58 % of ester of density 1.04 g/ml, which is fine
considering the starting materials are cheap.
This will be used to make triphenylmethanol by reaction with phenylmagnesium bromide.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I made some nitric acid from hardware store chemicals. I haven't titrated it yet, but I'm guessing it is about 50% concentration.

When doing the distillation, I ran a PVC hose from the takeoff outside, and it's a good thing I did - when the potassium bisulfate started to
crystallize, it almost all crystallized at once, giving off a lot of heat and overwhelming the condenser, sending boiling hot nitric acid vapors
through the hose. I'd never done a nitric acid distillation on this scale before, but I suspected that might happen, so I was prepared.
I didn't get a chance to finish my barium hydroxide because the nitric acid distillation took too much of my attention, but hopefully, I'll get to
that tomorrow.
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
Does anyone know what happened to the old thread? I found a link to it but apparently it "doens't exist".
Anyway, today I made some 2,3-dimethoxybenzaldehyde from ortho-vanillin and methyl iodide, with potassium carbonate in acetone. Then I condensed the
product with 2-cyanoacetamide to give 2-(2,3-dimethoxybenzylidene)-2-cyanoacetamide as nice white needles.
Interestingly the product has strong blue fluorescence under long wave UV light. With the 3,4-dimethoxy pattern, the fluorescence in turqoise instead.
And with the 4-dimethylamino pattern, the fluorescence in bright orange. I'll probably make a thread at some point with more details.
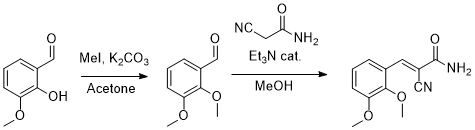
|
|
|
CouchHatter
Hazard to Others
  
Posts: 152
Registered: 28-10-2017
Location: Oklahoma
Member Is Offline
Mood: 76 elements taken!
|
|
About 2 months ago somebody had a topic about disappearing threads and falling post counts... Shortly after that the Everyday Science thread
disappeared, along with a handful or more other threads. It was probably missed quickly because of its sticky status.
In the OP, no one seemed to know what was going on, but it wasn't exactly the mod lounge. I remember JJay or jsum_1, streety or symboom, aga, maybe
coppercone were all there. I can't find it anymore, maybe deleted because I found a duplicate thread from 2015. Pretty sure its simply something to
deal with until the forum software is upgraded.
I'm moving into a new lab space! so I'm not exactly working every day on chemistry. Or any day for now. 
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I don't know what happened to it, and I'm not going to speculate. I had probably at least 50-100 posts on that thread.
I did not do any chemistry today because my Internet was down for much of the day, and I didn't have the necessary references. But I will be back at
it in full force tomorrow.
|
|
|
arkoma
Redneck Overlord
      
Posts: 1763
Registered: 3-2-2014
Location: On a Big Blue Marble hurtling through space
Member Is Offline
Mood: украї́нська
|
|
Quote: Originally posted by j_sum1  |
I am half-way through purifying some dioxane I made last night. I managed to get the tarry mess at the end of the synth to boil over and flood the
condenser. Twice. |
That is some tarry ass stuff too, but amazingly comes out of the glass easy. Finally bought a small (100ml) boiling flask for the pursuit of my
esters.
Medical hassles keep me out of the lab, kids are grown! (In hospital right now, just had surgery. Again *sigh*)
"We believe the knowledge and cultural heritage of mankind should be accessible to all people around the world, regardless of their wealth, social
status, nationality, citizenship, etc" z-lib
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I made a nice little batch of chloroform with the last of my bleaching powder in three runs. I don't recommend doing this unless you have a surplus of
bleaching powder, but you can add 100 mL of acetone to 300 grams of standard pool grade calcium hypochlorite and 1.5L water in a 3L flask in an ice
bath with mechanical stirring with a reflux condenser (add acetone slowly or the mixture might boil and foam excessively, filling the condenser with
suds. if that happens, dump ice water through the condenser to avoid making a big mess). Once the reaction stops, you can distill off the chloroform
on a water bath. It is possible to do the reaction without mechanical stirring by shaking the flask and cooling it repeatedly until shaking doesn't
make it warm up, but that is an enormous pain.
The crude product (which is what I have here) will be wet and probably contaminated with acetone. I'm going to wash mine with concentrated sodium
bisulfite solution, dry it over potassium carbonate, distill it, stabilize it with 1% ethanol, and store it with the rest of the lab chloroform.

Now that I've used up the last of my calcium hypochlorite, I think I'm going to stick with making chloroform with chilled liquid bleach. It's much
less work that way.
[Edited on 20-8-2018 by JJay]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Earlier I set up a hot filtration apparatus and did a dry run (I've never actually done a hot filtration before). Right now I'm boiling down some
crude barium hydroxide solution. It likely contains a little less than a mole of barium hydroxide, so I'm going to try to get the volume down to about
200 mL then filter and refrigerate. I'm hoping for white crystals. I have a bucket filled with dilute sulfuric acid that contains the waste and the
paper towels used to do the cleanup.
Oh and I'm also running a fractional distillation and slowly evaporating a solution on a steam bath.

[Edited on 31-8-2018 by JJay]
|
|
|
Heptylene
Hazard to Others
  
Posts: 319
Registered: 22-10-2016
Member Is Offline
Mood: No Mood
|
|
I made 400 grams of 60 % nitric acid by distillation from sodium nitrate and sulfuric acid. I tried to increase the strength of the acid to reach
around 65-70 %, but this seems impossible without a fractionating column.
The product will probably be used to make some o- and p-nitrotoluene.
I'm also purifying cinnamaldehyde from cinnamon bark oil using sodium metabisulfite. The goal is to form the bisulfite adduct, remove the rest of the
organics by filtration and the deprotect the cinnamaldehyde using a base. I wish I had an overhead stirrer because the bisulfite adduct forms a thick
slurry that is difficult to stir.
I'll post a thread when I'm done.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I did a hot filtration of crude barium hydroxide solution and then washed the filter cake and beaker with about 100 mL of boiling water.
Unfortunately, a small amount of silty material (likely barium carbonate) made it through the filter paper and cotton ball. Right now the solution is
cooling. I'm hoping that the silty material will go through the filter paper just as easily when I filter off the barium hydroxide, but I imagine I'll
have to recrystallize.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Turned some manganese sulfate into manganese carbonate
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Getting ready to run some distillations....

|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I made a slurry in water and added HCl and it's a chloride now.
one cool thing is that after additions of HCl, when the effervescence died down, there was a clear stratification of the translucent acidified layer
and the murky lower layer still containing suspended carbonate. There seemed to be points of reaction between the two, visible as a spot of bubbling
on the dark surface. They seemed to have some interesting dynamics: some of these upwelling spots persisted for a while, some extinguished quickly,
and some grew into a large foaming for a moment
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
I've been converting oxalic acid to formic acid all weekend. It's a cute little reaction cycle catalyzed by glycerine, in which a glyceryl oxalate
ester is formed, the ester is decarboxylated to a glyceryl formate ester, and the ester is hydrolyzed and the formic acid distilled off. Several
literature sources call for thorough drying of the glycerine beforehand, but it's not obvious why, and NurdRage didn't make any special mention:
https://erowid.org/archive/rhodium/chemistry/formic.acid.htm...
https://www.youtube.com/watch?v=ceL-I0azPH8 (with a guest appearance from Gary the Bug)
Anyway, I'm coming up on ~100 mL of distinctly anty-smelling crude product
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I've been thinking about doing that too. I have to wonder if there isn't an easier way, though... something like hydrogen peroxide and methanol....
Oh and I've been purifying solvents all weekend. I finally managed to clean my sep funnel by putting some abrasive cleaner and a handful of coins into
it and swirling it around. There was a waxy coating clinging to the walls, and now the funnel has that sparkling new glassware look to it.
[Edited on 23-9-2018 by JJay]
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Currently dissolving a neodymium magnet in sulfuric acid. Also made a few mL of crude methyl salicylate. Also mixed 50 mL of a lacquer thinner with 50
mL of water to determine the toluene/DCM concentration. Will start separating the components of the thinner next week when I have time, as toluene is
unfortunately not sold as a pure product anymore where I live.
The philosophy of one century is the common sense of the next.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
That's what i'm doing now - I had 20L of various waste solvents, most of which mixtures of methanol/water eluent waste from my HPLC.
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
Screwed up and made a pretty hefty amount of bismuth oxynitrate. Went and hydrolyzed my bismuth (III) nitrate I'd made by dissolving metallic bismuth
in nitric acid.
Damn. Would dissolving it in concentrated HNO3 push the equilibrium back the other way and allow me to recover the nitrate?
Edit: I believe my new strategy could be to dissolve it in weak nitric acid solution, and neutralize with sodium carbonate to get bismuth (iii) oxide,
followed by digestion in HCl and reduction with aluminum to yield metallic powdered bismuth, filtered, added to conc. nitric acid, and evaporated.
Phew! That's one heck of a round-trip if I can't just add it back to conc. nitric acid....
[Edited on 25-9-2018 by weilawei]
|
|
|
Daffodile
Hazard to Others
  
Posts: 167
Registered: 7-3-2016
Location: Highways of Valhalla
Member Is Offline
Mood: Riding eternal
|
|
Anyone on here ever thought about making some Xylyl Bromide? Found some Sodium Bromide solution at the store the other day, when I ran Chlorine
through it I got some nice dark droplets of Bromine. I destroyed that sample obviously but am considering making some Xylyl Bromide by just mixing
Xylene/ Bromine in reflux and irradiating (patent says a lamp should be sufficient).
I know that there will be a couple brain cells lost in the experiment but it seems like a pretty interesting chemical overall. I've made and tested a
couple other similar chemicals before and had a lot of fun, like chloropicrin or iodoacetone.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I removed a couple of centimeters from my 20 cm thermometer tube so that it will fit into a 3 L flask. I am not very experienced at glassblowing, but
I managed to get the end to seal without doing too bad of a hack job.
I also constructed a large gravity filtration apparatus by drilling numerous small holes into the bottom of a 3.75 L ice cream bucket. The bottom of
the pail is exactly the same size as a coffee filter. It works pretty well except the bucket is a little too flexible to hang without the sides caving
in and reducing capacity. I'm going to try it with a more rigid pail.
|
|
|
ninhydric1
Hazard to Others
  
Posts: 345
Registered: 21-4-2017
Location: Western US
Member Is Offline
Mood: Bleached
|
|
Just distilled out DCM and toluene out of a refinisher. Will do a fractional distillation when I have time to separatye the two.
The philosophy of one century is the common sense of the next.
|
|
|
Sulaiman
International Hazard
    
Posts: 3730
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Quote: Originally posted by JJay  | | I also constructed a large gravity filtration apparatus by drilling numerous small holes into the bottom of a 3.75 L ice cream bucket. The bottom of
the pail is exactly the same size as a coffee filter. It works pretty well except the bucket is a little too flexible to hang without the sides caving
in and reducing capacity. I'm going to try it with a more rigid pail. |
For my diy heating mantle I bought some glass fibre cloth
https://www.ebay.co.uk/itm/0-03mm-Ultra-Thin-Fiber-Glass-Fab...
It is very fine mesh, it has unbroken threads / no loose bits, it is surprisingly strong, and resistant to heat, acids etc.
I think that it may make an excellent filter material,
with or without mechanical support.
It also looks suitable as a simple mechanical membrane for an electrochemical cell.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
It worked OK, but it still takes forever to filter calcium salts.
I was thinking about doing that with some ceramic paper, but it's a little on the expensive side to use for filters... interesting idea, though.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I put some more solvents in bottles. I probably ought to recycle my waste toluene.... At some point today, I plan to recrystallize some sodium
carbonate.
|
|
|
| Pages:
1
2
3
4
..
14 |