| Pages:
1
2
3 |
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
disulfur dichloride
Some time ago, I tried out the synthesis of "Chlorschwefel" (S2Cl2).
The setup was a simple distillation apparatus.
The distillation flask contained 35g of powdered sulfur. A tube was inserted into the stillhead down into the sulfur. It was attached to a chlorine
generator.
The sulfur was heated until it was molten and began to darken and get viscous.
Chlorine was bubbled through the sulfur, while continuing the heating.
It was going fine and smooth, a red liquid was distilling over.
At the end I had about 20ml of a red fuming liquid in the receiving flask.
I had to stop the process before all the sulfur was consumed because tere was a leak in the tubing that let out chlorine and I was coughing badly.
Things I learned:
- bleach + HCl is not a good way to prepare chlorine, it doesn't produce much Cl2 and a lot of it dissolves in the large volume of aqueous
liquid.
- Calcium hypochlorite + HCl works much better, it liberates a lot more Cl2.
- the sulfur must be really hot to react fast enough, when the sulfur is just barely molten a considerable fraction of the Cl2 passes through it
without reacting.
- bubbling Cl2 through molten sulfur is a feasible route to S2Cl2.
My distillate contains a lot of SCl2 because it is red, pure S2Cl2 is yellow. I will have to redistill it with sulfur. (SCl2 reacts with sulfur to
S2Cl2)
I know that there is already a thread about sulfur chlorides, but it discussed a different method of preparation and it is also very old. (about 2
years)
The S2Cl2 smells indeed bad, but the stench is not unbearable. I have prepared substances (diethyl sulfide) that smell MUCH worse.
S2Cl2 reacts rather slowly with water, as I saw. The blob stays in the water for some time before decomposing into sulfur, SO2 and HCl.
It should be noted that I'm now only one reaction away from sulfur mustard, although I will surely never try to make it.
I hope this synthesis is of interest for you. S2Cl2 is interesting because it can dissolve up to 67% sulfur. It also dissolves white phosphorus and is
therefore an ideal substitute for CS2. It's also non- flammable.
|
|
|
BromicAcid
International Hazard
    
Posts: 3264
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
One thing I wonder, what kind of distillation apparatus did you have?
During my attempts at distilling CS2 from a mixture of carbon and sulfur as my product came over it coated my vessel and my condenser and everything
else in fine sulfur that was very very very very very very hard and very very very annoying to remove. Does distilling this sulfur and chlorine
mixture cause the same carryover of sulfur and subsequent precipitation onto all glass surfaces?
[Edited on 9/4/2004 by BromicAcid]
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Those (S2Cl2 and SCl2) being compounds of divalent S, I am somwhat surprised that the reaction with an excess of Cl2 gas did not also produce SCl4 and
S2Cl4 and S2Cl6, compounds of tetravalent S. (SCl6 may not exist for steric reasons, despite the existence of very stable SF6 in which the S is
tightly surrounded).
John W.
|
|
|
BromicAcid
International Hazard
    
Posts: 3264
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Surprised it didn't form SCl4? Do you know the properties of SCl4? If you knew the properties of this gas you wouldn't be surprised at all,
it decomposes at -38C to form SCl2 and Cl2 and therefore at the temperatures involved it would be a moot point. In addition SCl6, as you predicted
does not exist, Cl2 is not electronegative enough to pull off the +6 oxidation state of sulfur and as you said steric hindrances would put a damper on
the already strained molecule.
As for disulfur tetrachloride, doing a google search for it brought about 4 results, I would assume it is even more unstable then sulfur
tetrachloride based on that.
[Edited on 9/4/2004 by BromicAcid]
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I used the following apparatus:
an 100ml rb flask with a claisen adapter attached to a liebig condenser and a receiver flask. The chlorine supply line was inserted into the first arm
of the claisen (down into the sulfur) and a thermometer was in the second arm.
The chlorine supply line was inserted airtight (cork+teflon tape) into the claisen.
Yes, after the reaction my entire apparatus was covered with fine sulfur dust on the inside.
After I dismantled the apparatus, the residual S2Cl2 in the apparatus hydrolized with air moisture and formed more fine sulfur.
This was a real pain to get off. It ist't even clean yet.
Well, I just have to distill some 99% nitric acid (from NaNO3 + H2SO4) to clean it. (99% HNO3 oxidises sulfur to sulfuric acid at room temp.)
|
|
|
BromicAcid
International Hazard
    
Posts: 3264
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Does the S2Cl2 that came over have much sulfur dissolved in it? Have you attempted to evaporate it and see how much residue it leaves?
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
It left NO sulfur upon evaporating.
Even when I dissolved some sulfur in it, it still left no residue. 
This was because it contained lots of SCl2 which reacted with the sulfur to form S2Cl2.
S2Cl2 is yellow while SCl2 is red.
And my product was deep red when it came over.
If I wanted pure S2Cl2 I would add sulfur until the liquid is yellow.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by BromicAcid
Surprised it didn't form SCl4? Do you know the properties of SCl4? If you knew the properties of this gas you wouldn't be surprised at all,
it decomposes at -38C to form SCl2 and Cl2 and therefore at the temperatures involved it would be a moot point. In addition SCl6, as you predicted
does not exist, Cl2 is not electronegative enough to pull off the +6 oxidation state of sulfur and as you said steric hindrances would put a damper on
the already strained molecule.(cut) |
The non-existence of SCl6, unlike SF6, cannot be due to redox or ionization-potential reasons, as the S(VI) oxychloride SO2Cl2 exists (melts at
-54.1ºC, boils at 69.1ºC). It MUST be steric reasons.
The data I have e.g. from Perry says that SCl4 melts at -30ºC and decomposes at under -20ºC. This is surprising (I did not think of looking up SCl4
as I thought it should be more stable), as neither S(IV) nor S(VI) compounds are regarded as being oxidizing, and SO2 is a moderate reducing agent.
Steric factors may be the explanation for SCl4's easy decomposition - the 4 Cls plus the unused pair of p electrons on S should be at the apices
of a trigonal bipyramid, with the Cls changing places (in the liquid phase) so as to be equivalent.
John W.
|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
Oh, maybe I should've searched for this thread before attempting to make S2Cl2...
Those of you that did this experiment, how do you avoid S2Cl2 decomposing to H2S?
I found this quite annoying while washing the apparatus.. thoat that NaOH(aq) would retain H2S formation but it didn't..
Also, I didn't immerce the gas inlet tube under sulfur as was recommended by two nonorg books.. (it was about 1,5 cm above S)
That was probably the main reason why my exhaust gas filtering train was overloaded.. both washing flasks with Na2CO3(aq) was filled with green Cl2
and coal filter was hot.. 
As for results, I got a crap yield. It could be because I used stochiometric amount of Cl2 or simply because of incompleate reaction.
[Edited on 12-8-2005 by frogfot]
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
You definetly want the tube well below the surface of the molten sulfur, as deep as possible. You also want the sulfur extremely hot, as hot as
possible (less than 400C, I *think* 300C seems about right where I got good yields, didnt have a thermometer in it). High temp also reduces the
amount of chlorine, although a very large excess is still needed. I wouldnt even bother to measure the amount of Cl2, just pump in a lot.
As for gas scrubbing, I had good success with just 1500mL of water with ~230g of NaOH dissolved in it and cooled.
Im not sure why you would be getting H2S, are you really sure about that? Make sure your chlorine is absolutely dry (a simple CaCl2 tube works very
well), the sulfur should be too.
On a personal note I haven't been messing with chlorides and oxychlorides of sulfur in order to give myself some time to recover. They are a lot
of fun and very interesting to play with, but the proper precautions should be taken. (I know, I'm preachin to the choir here, it's as much
for me as anyone else.) 
Also check out this thread, which is about thionyl chloride but varies in topic on many chlorides and oxychlorides of sulfur.
http://www.sciencemadness.org/talk/viewthread.php?tid=1439&a...
Nature's Natrium
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
hinz
Hazard to Others
  
Posts: 200
Registered: 29-10-2004
Member Is Offline
Mood: No Mood
|
|
I passed the chlorine through not too hot sulfur (not above 120°C) and not too fast, then nearly all the chlorine gets reacted. The greatest problem
when making SCl2/S2Cl2 is, that it will kill nearly all tubes I had. Silicone which is resistent aggainst chlorine will crack ( normally it's
flexible, but when it comes in contact with sulfur chlorides, there will be cracks and you only have to touch it an it falls apart)
Later I used PVC to collect the SCl2( b.p. 59,6°C Brauer) vapors and the tube was not too much attacked, but I had to change it after a half an hour
or so. When the temperature is to high, the SCl2 vapour is too hot and will decompose the tubes faster. My setup were one big chlorine gererator (
bleach + HCl ) two sauce bottles filled with conc. H2SO4 and a third sauce bottle filled with sulfur which got heated in an oil bath on 120°C. From
there a fourth bottle was connnected with PVC tube which collected the SCl2 vapour which condenses in the tube. The last tube and the last bottle got
cooled in an ice bath. The sauce bottles had an lead stopper ( self casted ) which was warped with teflon tape. Trough the stopper I made two holes
trough which I sticked two glass tubes. The apperatures were more or less tight, later I removed one wash bottle which was untight. Yields of the
synthesis are i the attachment, athough it could be greater I blame the bad chlorine generator (dissolved chlorine in the water) It was half a year
ago my equpment improved till now
P.S. If you plan to make sulfur mustard, don't use the H2SO4 / ethyl alcohol method, make your ethene by passing ethanol vapour through hot
(450°C) beta Al2O3 ( got from DC chromatographie plates ) and dry it afterwards. With the sulfuric acid method I only got lots of dehydrathed alcohol
(black ugly smelling(SO2) mix of carbon and sulfuric acid, I took the wole pot and put it outside) It was a waste of sulfuric acid

|
|
|
frogfot
Hazard to Others
  
Posts: 212
Registered: 30-11-2002
Location: Sweden
Member Is Offline
Mood: happy
|
|
| Quote: |
Im not sure why you would be getting H2S, are you really sure about that? Make sure your chlorine is absolutely dry (a simple CaCl2 tube works very
well), the sulfur should be too.
|
It actually didn't smell like H2S use to.. it smelled like sewer waters... in lack of better approximation...
Some MSDS for S2Cl2 mentions H2S formation too:
http://www.ilo.org/public/english/protection/safework/cis/pr...
"The substance decomposes on heating or on burning
producing toxic and corrosive fumes including hydrogen
chloride, hydrogen sulfide, sulfur oxides."
Btw, litterature recommends 125-130*C.. 300*C seems to be alot.. 
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by frogfot
Btw, litterature recommends 125-130*C.. 300*C seems to be alot.. 
|
Well, it's entirely possible it works (better or worse?) at a lower temp. I know that I, based on the advice of garage chemist, heated the
sulfur up very hot, and the reaction did work. I suppose further inquiries into the optimal temp for this reaction are called for, although it will
be some time before I get around to attempting this again. Maybe you can elucidate the situation, frogfot?  
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
garage chemist
chemical wizard
    
Posts: 1803
Registered: 16-8-2004
Location: Germany
Member Is Offline
Mood: No Mood
|
|
I do NOT plan on making sulfur mustard. It is useless in preparative chemistry and of absolutely no interest for me.
Just wanted to clarify this.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
illustration
FWIW, this is from one of the books that I only scanned partially, Lab. Meth. Inorg. Chem. by Biltz, Hall, and Blanchard, since it is otherwise
unavailable.
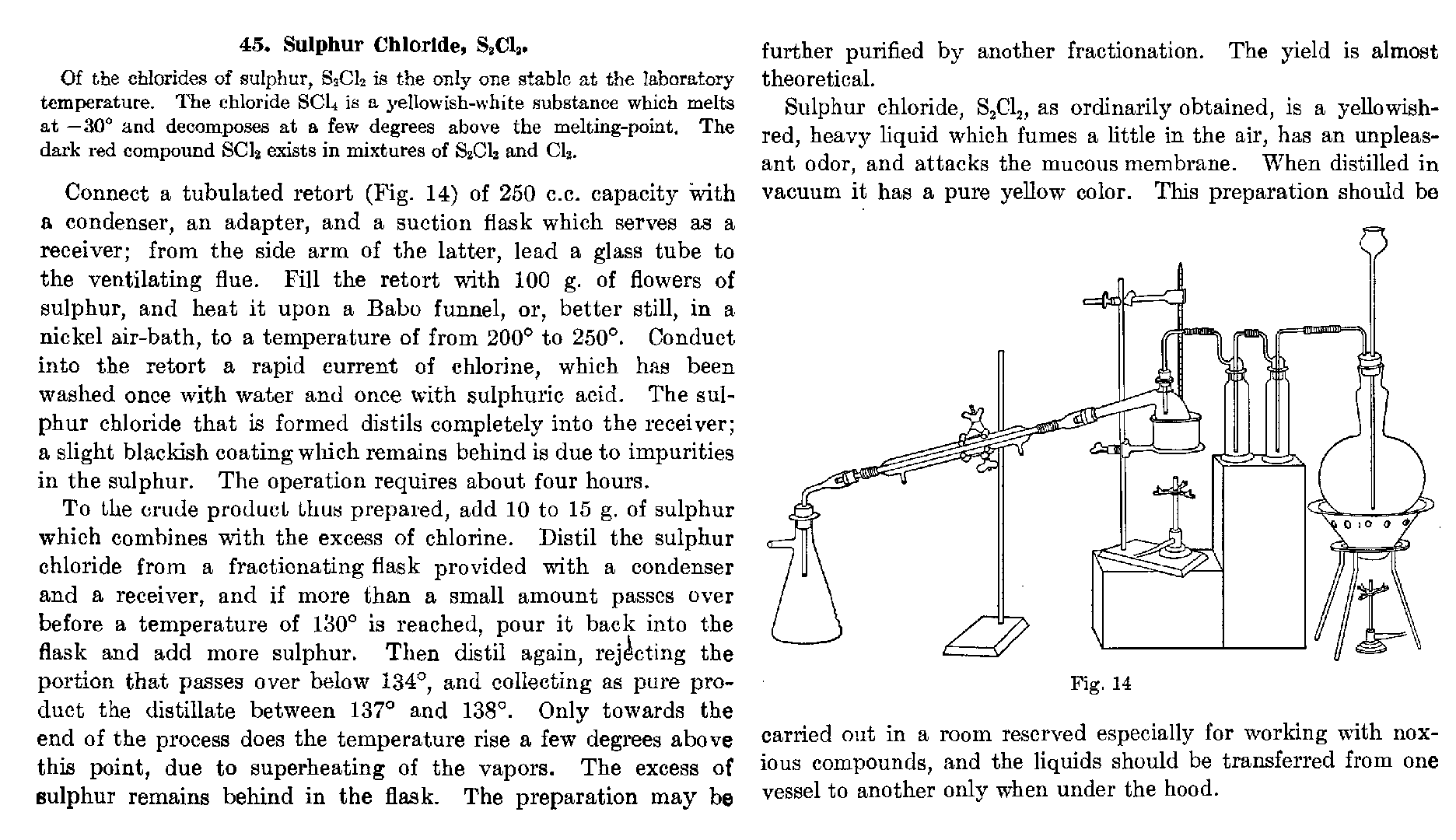
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Would adding C3Cl3N3O3 (TCCA) to molten sulfur work to make a sulfur chloride? if the reaction worked it should make CO and N2 i would think along
with the sulfur chlorides like this: 2C3Cl3N3O3 + 6S = 3S2Cl2 + 3N2 + 6CO
the melting point of sulfur is 112.8C
and the decomposition point is 225C for TCCA, its melting point is 249.00 - 251.00 deg C
can anyone see a good reason why this will not work or a good reason why it will? if this works it will be a lot funner to make S2CL2 or SCl2
[Edited on 30-10-2005 by kclo4]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Surely you would get a reaction if you mix a weak reducent like sulphur with such a strong oxidant like TCCA. The reaction would certainly escape
control though and without a fume hood and proper protection you would likely poison and/or burn yourself. I once tried to oxidize sulphur in acetic
acid with TCCA in order to get acetic anhydride trough in situ SOCl2 formation. Well, it did not yield any dehydrating species able to form the
anhydride but the reaction was extremely exothermic and TCCA had to be added very carefully trough the reflux condenser in very small portions (a lot
of nasty fumes evolved). Besides the usual cyanuric acid precipitate the acetic acid contained an intensively yellow product, probably some undefined
mixture of nasty stuff that might have been anything from various sulphur chlorides to something like ClSCN which decomposes further.
But in no case the reaction equation can be like you wrote it down. Sulphur can’t reduce TCCA all the way to carbon monoxide (CO), it is simply
impossible. In theory (if the reaction would be tempered by a solvent and cooling) one would get ClSCN or its trimer and soon after its decomposition
products. Don’t ask me what are the decomposition products of chlorothiocyanate since I’m not an inorganic chemist. 
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
......Wiat are you saying that S2Cl2 or SCL2 will not form or did i read it wrong?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm saying that I do not know what exactly would form (except that CO would not form). I warned you about the dangers of heating a
mixture of TCCA with sulphur and gave you a hint on how to figure out the end products (the decomposition products of ClSCN).
(that is what I wrote in few words)
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Illegal Parkinson
Hazard to Self
 
Posts: 85
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
That blood-red attempted S2Cl2 looks interesting. Also regards to SCWack for the upload. This is covered in "Kings Chemistry" as well
although I guess what you guys really want is first-hand info and not text-book stuff. I would be interested in this synth myself. However I missed a
good opportunity to get a couple of gas dispersion tubes yesterday. I guess i'll have to put the liquid Cl2 generator off for a little while
longer.
|
|
|
Douchermann
Hazard to Others
  
Posts: 117
Registered: 11-10-2005
Location: Illinois, USA
Member Is Offline
Mood: No Mood
|
|
Would anyone happen to know if SxCl2 would attack copper or brass tubing pipes/fittings?
It\'s better to be pissed off than to be pissed on.
|
|
|
kclo4
National Hazard
   
Posts: 916
Registered: 11-12-2004
Location:
Member Is Offline
Mood: No Mood
|
|
Well... SCl2 would be in there, and doesnt that decompose letting off chlorine when heated? Also if sulfur is added to the SxCl2 it will turn to S2Cl2
with a bit of heating.
So the copper could maybe reduce it at room temp or hotter, but i dont know!
but i think it would yes
|
|
|
Bolt
Hazard to Others
  
Posts: 188
Registered: 26-3-2007
Member Is Offline
Mood: No Mood
|
|
US2871098A Production of hydrogen chloride and sulfuric acid [chlorosulfonic acid]
Attachment: US2871098A Production of hydrogen chloride and sulfuric acid [chlorosulfonic acid].pdf (526kB)
This file has been downloaded 1034 times
|
|
|
peach
Bon Vivant
    
Posts: 1428
Registered: 14-11-2008
Member Is Offline
Mood: No Mood
|
|
{this copypasta from a post I made on another forum, but something is messing with the Madness mind and the formatting has gone funny}
Which chloride?
Initially, sulphur will form disulphur dichloride (S2Cl2) - this is sometimes called "monochloride", but I prefer "di-di" as a reminder that there are
two of each.
Yellow liquid
135.04 g/mol
-80C MP
137.1C BP
1.688 g/cm^3
Decomposes in water with loss of HCl
Soluble in ethanol, benzene, ether, chloroform and carbon tetrachloride
Interesting transient property - it converts to S=SCl2 under UV radiation
When additional chlorine is available, it will carry on to sulphur dichloride.
Cherry red liquid
102.97 g/mol
-121.0C MP
59C BP (Decomposing)
1.621 g/cm^3
Hydrolyses in water
Indeed, it will continue on either further towards SCl4; which is unstable at room temperature.
As it turns out, this molten sulphur method I will first look at makes it difficult to produce one specific chloride straight off. The result will
likely need retreating if you require specifically the "di-di" or sulphur chloride rather than a possible mix of the two. I'll discuss how to do this
further down.
Another method to di-di
Is from the chlorination of carbon disulphide. As this is a practical forum, and very few people will have CS2, I will avoid that one for now.
Decomposition
As it is quite simple to move between the chlorides and there are a variety of them, their decomposition is not so straight forward.
[QUOTE]"When heated to decomposition sulphur dichloride emits fumes of sulfur oxides and hydrogen chloride. Decomposition into disulfur dichloride and
chlorine at high temp. Decomposes into hydrogen and chlorine hydrochloric acid on contact with water."[/QUOTE]
- an MSD for sulphur dichloride from Chemicalbook.com, who's grammar I have edited a little.
[QUOTE]Disulphur dichloride reacts slowly with water to produce a complex mixture of things including hydrochloric acid, sulphur, hydrogen sulphide
and various sulphur-containing acids and anions (negative ions). There is no way that you can write a single equation for this - and one would never
be expected in an exam.[/QUOTE]
- from ChemicalGuide.co.uk
Interestingly, the same article mentions;
[QUOTE]Disulphur dichloride is just one of three sulphur chlorides, but is the only one mentioned by any of the UK A level syllabuses. This is
possibly because it is the one which is formed when chlorine reacts with hot sulphur.[/QUOTE]
- from ChemicalGuide.co.uk
This, as you'll see, is not entirely true, and adds yet another facet to dealing with it.
[QUOTE]Disulphur dichloride is a simple covalent liquid - orange and smelly![/QUOTE]
- from Chemicalguide.co.uk
This is correct, particularly the last point!
What is sulphur dichloride useful for?
[QUOTE]SCl2 is used in organic synthesis. It adds to alkenes to give chloride-substituted thioethers. Illustrative applications are its addition to
1,5-cyclooctadiene to give a bicyclic thioether and ethylene to give sulfur mustard S(CH2CH2Cl)2.
SCl2 is also a precursor to several inorganic sulfur compounds. Treatment with fluoride salts gives SF4. Reaction with ammonia affords sulfur nitrides
related to S4N4. With H2S, SCl2 reacts to give "lower" sulfanes such as S3H2.[/QUOTE]
- Wikipedia
And the di-di?
[QUOTE]S2Cl2 has been used to introduce C-S bonds. In the presence of AlCl3, S2Cl2 reacts with benzene to give diphenyl sulfide:
S2Cl2 + C6H6 → (C6H5)2S + 2 HCl + 1/8 S8
Anilines react with S2Cl2 in the presence of NaOH via the so-called Herz reaction to give ortho-aminothiophenolates. These species are precursors to
thioindigo dyes. It is also used to prepare the sulfur mustard "gas" by reaction with ethylene:
S2Cl2 + 2 C2H4 → (ClC2H4)2S + 1/8 S8
Other uses include manufacturing sulfur dyes, insecticides, synthetic rubbers. Also used in cold vulcanization of rubbers, as polymerization catalyst
for vegetable oils and for hardening soft woods.[/QUOTE]
- Wikipedia
Anything else exciting
Yes, there is.
Of all the methods to thionyl chloride, that which treats the sulphur chlorides with sulphur trioxide is apparently the most practical.
Others require either the use of phosphorus, phosgene or sub-zero temperatures to liquefy gases, and temperatures that are just beyond those you can
easily achieve. The only other option is using sulphur dioxide, trioxide and chlorine in a homogeneous vapour method.
None of these are particularly appealing to me at present, which is part of my interest in obtaining the sulphur chlorides.
Whilst it is not really the subject of this thread (and I don't want this thread to become one about thionyl chloride - I will start another), I will
mention that thionyl chloride can be made from either the di-di or sulphur dichloride upon treatment with tioxide.
My predictions
I was planning on making the di-di form by slow bubbling, thinking the sulphur chloride Klute obtained may be due to his rate being high enough to
push the orange / red sulphur chloride over below it's boiling point.
This means I based my gas generator on the volume of chlorine needed to do that, adding some excess on to account for the generator not reaching it's
full yield and possible poor absorption.
My predictions about Klute's result were incorrect and I also obtained some sulphur dichloride.
The formulae
| Quote: |
S8 + 4 Cl2 → 4 S2Cl2; ΔH = −58.2 kJ/mol
S2Cl2 + Cl2 → 2 SCl2; ΔH = −40.6 kJ/mol
|
- Wikipedia
Let's get some pictures happening!
I will be using a 50ml flask. I spent a while wondering if I could simply use a gas inlet for the chlorine, worried that bubbling chlorine through
might encourage sulphur to pass over with the product. In the end I went with the tube in the sulphur idea and found this worked perfectly well. To
obtain a blow pipe, I will butcher this rarely used Thistle funnel.

I've scratched and snapped it down to an appropriate length and then flame polished the end to a small, angled point (allowing it to poke as close to
the base of the flask as possible).

Tremble before my glass blowing skills! 

I am giving the glassware a warm in the microwave to drive off any water and it'll then be rinsed out with some acetone. Don't include any plastic
fittings, as they get hot almost immediately even on low settings. Be wary of heating chemicals, or any traces of them (like KOH from cleaning) in the
microwave, as they can spatter and become extremely hot. I have had a turn table platter burst when opening the door to one, shooting finger sized
bits of glass literally across the room. Stand to the side of the door when opening and allow at least thirty seconds before assuming it's safe to
reach in (get on with something else in the mean time).

I'll be using this pet sulphur as the source. I think I trust the pet sulphur more than I do bags of powdered chemicals from eBay, as this stick has
clearly been produced industrially and is not a gardening product. It's also harder to cut solid objects with bulking agents (which is a genuine risk
when buying repacked materials).

The sulphur is starting to warm up. In the meantime, I'm setting up the generator and other bits and pieces.

100g of "99% min assay, high purity manganese dioxide" from eBay. I will be using an excess of this, as I am not 100% about that 99% figure, so I will
use my hydrochloric acid to set the gas volume.

The sulphur is molten, things are getting underway.

The generator.

I'll be taking the chlorine off from the top of the funnel. I have two funnels on here, each with around 100ml of hydrochloric acid in them.

Why the top? Because this sulphuric acid drying stage is next. Once the funnel is empty, I can close the tap. Should anything from a subsequent stage
be sucked back (which can happen very easily and unpredictably with these), it'll go into the closed off funnel, not straight into the generator it's
self.

The gas enters the sulphur through the blow pipe.

And leaves through a silica gel tube that is there to prevent moisture being so easily pulled from the atmosphere back to the receiver, which will
encourage hydrolysis of the products. I couldn't predict how much they'd want to hydrolyse (which isn't much), so could have used anything from
nothing to everything on the exit. Since the silica tube is easy, I went with that. The constant flow of chlorine alone helps prevent moisture back
tracking. I also thought about adding absorption traps to collect chlorine and other harmful gases from the experiment.
The generator is capable of raising this room's Cl2 concentration to >300ppm, which is approaching an immediately threatening concentration and
there are other possible risks from hydrolysis products. However, I have a brand new full face mask and we can smell chlorine at just 3ppm. Adding
absorption traps would mean adding a lot more between them and the flask to ensure the product wasn't exposed to an even better source of water.
Again, not having done this before or finding any information about it, I opted to avoid the extra complexity. I'd simply turn the generator off, put
the mask on and add them if it became a problem. In reality, not only was not a lot of any harmful gas released, I did not smell chloride, sulphur,
sulphur dioxide, hydrogen sulphide or any other risk you might associate with this, at all, through out the entire experiment. The
hot sulphur must do a good job of soaking it up.

The whole.

The chlorine generator, slowly bubbling away. It's about 10 to 15C out here.

Chlorine building up in the glass.

The experiment is now running. As sulphur boils higher than my mantle can reach (444.6C), I was able to turn it up towards the high end (300C; it's
supposed to reach 350C I think but that's pushing the realms on practical reality). One thing I was concerned about was that there is gas moving
through the still. If you blow shield gas over a volatile solvent (even at room temperature), you will find it making it's way out of
the exhaust quite rapidly.
I thought, if I push the mantle to the full setting, whilst the sulphur may not boil and will be reacting more rapidly, the chlorine flow may begin
picking it up and moving it through to the receiver. Instead, I began at the molten point and, over the hours, turned it up a little at a time.
Watching for any signs of gross crossover; e.g. the still head rocketing up towards the BP of sulphur. It wouldn't reach that boiling point, but it'd
be well above that of the chlorides.
I could have stuck a thermometer in there I guess, if I had one spare, and a spare adaptor, but this was more a test of the rough idea and you can
always donate some money for those extra numbers in the future. Suffice it to say, I ran most of the experiment with the mantle at around 8 on the
dial, so it's probably around 250C.

That isn't sulphur on the glass, it's the chlorides. Which means you have to watch the still head temperature for contamination, not for something
that looks like sulphur on the glass.
The thermometer temperature would vary between 90 and 110C, which happens to be between the two points for the two chlorides. The liquid on the glass
is yellow, suggesting di-di, but the distillate soon turned orange and then red.

Many hours later, around 16 of them, I see a pleasing quantity of liquid appearing in the flask. From my bit of paper calculation, it looks close to
the full yield for di-di; but it's the wrong colour.

This is taking a long time. The reason being, the generator that is gradually slowing down. Even at it's full rate, it wasn't bubbling more than once
every second or so. I have now started warming it to push it along again. That it does for a few more hours, before the bubbling stops at almost
precisely 24h from the start.

I was hoping to get all the sulphur out to avoid having to clean it off, and to get some decent yield out of this. I discovered the next day, pretty
much all of it has gone. It took a lot of patience to wait for the generator to entirely cease.

The result. Holding it up to the light, you can see that is a nice cherry red; which means it's either contaminated with or all sulphur dichloride.




I'm going to recover the "high purity" manganese so I can clean it up and reuse it. I am also going to look at how pure it actually is by weighing
that component of it which will not dissolve in concentrated hydrochloric; as I do not trust eBay's version of "high purity" and suspect this may be
pottery grade or recovered battery paste (I have also heard of it being cut with sand). Rather than get the glassware dirty for this, it's time to
break out the clandestine plastic funnel and coffee filter papers. This filtrate is brown, so it at least has iron chloride in it. Needs another run
through some coffee papers to get the last of the solid out (double or triple them up and be careful of their seams, where they're most prone to
leaking particulates).


Once cooled, you can see almost all of that yellow has disappeared. It was chlorides, not sulphur. On rinsing the glass out, there are virtually no
signs of elemental sulphur left. The glass fumes a little when exposed to water, but not a whole lot when opened. It also develops a waxy opaque layer
and tacky feel to it. A decomposition product of di-di is elemental sulphur, which is probably largely what this opaqueness is.
I will use a hot air gun with the glass upside down to remove any elemental sulphur remains. Carbon Disulphide could also be used if you've got some
handy. Or stick it in the oven and burn it clean; the more universal cleaning method.


I am now weighing a 20ml sample of the result in an Emil Class A volumetric flask. My aim is to ascertain it's density.

An empty bottle it can live in.

And full.

Done. The sample was around room temperature, having been sat here for about an hour at 1012mBar.

Results
As you can see, that flask is not pure di-di.
So how has sulphur dichloride got into the results if it decomposes on boiling?
Likely because there is an uncontrollable excess of chlorine in this method. As the di-di leaves the still, it is still warm, and there is still
chlorine in the atmosphere around it. Allowing it to progress to sulphur dichloride.
The drips coming out of the still ended up cherry red early on. Indicating that this is happening further back as well, not just in the receiving
flask.
The odd still head temperature
I was somewhat expecting, due to the gas flow.
Sulphur Dichloride BP: 59C
Di-di BP: 137.1C
Mid point: 98C
My overservation, pretty much that mid point.
Density
A 20ml sample (+/- 0.03ml) weighs 33.16118g, giving a density of 1.658 g/cm^3.
Compare it with 1.688 g/cm^3 for the di-di and 1.621 g/cm^3 for sulphur dichloride.
The mid point between the two is 1.655 g/cm^3.
My density is just 0.2% off the middle of both.
Possible density error source - Sulphur in the distillate
The chlorides can dissolve sulphur to some extent and may have carried traces over with them, which could theoretically play with the densities
obtained.
Calculating maximum yields from the sulphur mass used
I didn't weigh the sulphur in very accurately, since I was intending more to see how feasible the reaction was rather than investigate it's precise
yields.
| Quote: | | S8 + 4 Cl2 → 4 S2Cl2; ΔH = −58.2 kJ/mol |
I used approximately 23.16g of sulphur; 0.72 moles, 12.73 ml when molten.
Assuming complete conversion, this gives 0.36 moles of di-di; 48.61g, 28.79ml.
Had the reaction progressed to complete conversion to sulphur dichloride;
[QUOTE]S2Cl2 + Cl2 → 2 SCl2; ΔH = −40.6 kJ/mol[/QUOTE]
I'd have 0.72 moles of sulphur dichloride; 74.13g, 45.73ml.
The mid point between these two is 61.37g, 37.26ml.
So how much DO you have?
I have 46.7877g of something with a density of 1.658 g/cm^3, giving me a volume of 28.22 ml
Checking against the chlorine source
All that can be said with regards to the chlorine is that there was an excess present to go onto the second stage of chlorination even if the entirety
of the sulphur had to first pass to di-di.
In practice, it doesn't all go to di-di and then to sulphur dichloride, both are forming simultaneously as they come off the molten sulphur and leave.
I do know the theoretical quantity of chlorine produced, but since I don't know the ratio by which the two stages share it in practice, I can't
estimate the quantities of each chloride in the receiver from this.
It would also be impossible to produce such a specific ratio, as it will vary with equipment, flow rates, temperatures, pressures and more.
Yield
Is impossible to accurately determine as there is almost certainly a blend of the two products, with significant quantities of each present.
However, based on me not being able to smell excess chlorine leaving and the various volumes and weights, this is a practically worthwhile method of
producing the chlorides.
Summary of evidence
Colour - Is certainly red (like sulphur dichloride), but di-di is yellow / orange so not a great indicator
Temperature - Came over at in a band between the two
Density - Almost exactly the mid point between the two
Conclusion
It works and I've run far more tedious experiments.
But as you can now see, you're quite unlikely to get solid evidence of which chloride you have.
It is also an unacceptably 'messy' method of producing them if a single chloride is what you're after, directly out of the still. You are not in
positive control of what is in excess or demand at the various points (from the molten sulphur, to the receiver). This either needs taking into
account when using the resulting liquid, it needs producing by the method I'll mention at the end (which I will attempt once my latest order of
solvents arrives) or the crude distillate needs tidying up.
Getting a pure result - clean up
| Quote: | | Disulfur dichloride, S2Cl2, is the most common impurity in SCl2. Separation of SCl2 from S2Cl2 is possible via distillation with PCl3 to form an
azeotrope of 99% purity |
- Wikipedia
There are many ways to skin a cat, and sometimes it doesn't involve phosphorus.
By redistilling this unknown mixture over sulphur, any SCl2 will decompose back to the more stable di-di.
Conversely, treating the distillate, now separate from excess sulphur, with chlorine will push it all towards sulphur dichloride. This is less stable
and so you would probably want to do it immediately before use.
You may also wish to vacuum degas both of them before use, as they both decompose around moisture, releasing hydrogen chloride and sulphur dioxide.
They can also contain spare chlorine. Other contaminants from production and decomposition would include elmental sulphur and oxides of sulphur.
Suggestions
Be very careful trusting gas generators if your product can't stop at a specific point.
Also, be careful assuming the materials are going to give you a true, full yield and that they are finished when the bubble rates slow.
And how does di-di and sulphur chloride smell?
Well, given that it's still a blend of things and will decompose into other blends of gases, it's quite hard to give it one particular smell. The
overwhelming smell is that of sulphur and chlorine.
It does have a slight smell of bacon and rotting, like hydrogen sulphide; but it is nowhere near as fierce as the latter.
How does it behave around the atmosphere?
My house has a humidity of about 40% RH most the time, which is about normal.
On opening the flask, there were some very faint fumes from it. But it does not fume profusely. It will appear to be doing not very much at all when
undisturbed.
The only times I noticed fuming was when it was disturbed. For example, when I was pipetting it out to determine the density. Once in the volumetric
flask, it was sitting quietly again.
Momentarily back to thionyl chloride
Both di-di and sulphur dichloride will react with sulphur trioxide to produce thionyl chloride.
But the cleanest and most efficient method, in my opinion, is to start with sulphur dichloride, as this simply involves combining the two to yield the
thionyl and sulphur dioxide as a by product. As this is a gas, the reaction is going to be driving it's self to completion and cleaning it's self up
as it goes.
Any remaining dissolved dioxide or trioxide would then leave when the thionyl is distilled.
When doing this with di-di, one of the by-products is sulphur, which will likely start making things more complex in terms of cleaning up the thionyl
chloride.
My future plans
This wasn't so bad to run. The the result is clearly all over the place in terms of the chlorination states.
It is usable for things like thionyl, raw, and it can be swapped from chloride to chloride by using the methods above to clean it up if need be.
But Grind claimed to have had great results using chloroform as a solvent.
The chloroform and sulphur go into a flask as I have done and chlorine in blown into the mixture under reflux.
The reflux is at 61.2C, below the melting point of the sulphur, but the chloroform can dissolve a small amount of sulphur and bring it into better
contact with the incoming chlorine.
In this arrangement, it is easy to check that there is a constant excess of sulphur, as it will be present as solid lumps, distinct from the solvent.
Under these conditions, the di-di form will be the predominant (if not sole) form produced.
It gradually builds in the solvent until the solid sulphur is nearing depletion or the solvent has reached saturation. At which point, the remaining
sulphur can be filtered off, the solvent removed and the product recovered. This is then distilled to remove the traces of sulphur that were dissolved
in the solvent. By doing this over some sulphur, you can be more sure again that the distillate is free of sulphur dichloride and is instead di-di.
Grind mentioned having done this with 200g of sulphur and that he yielded faint yellow product; indicating he has the di-di form.
It is quite likely this works with dichloromethane and carbon tetrachloride as well.
11g of sulphur will dissolve per litre of DCM. I believe this number is 16 to 17g for chloroform.
Those capable may wish to look at the paper;
SOLUBILITY VII. SOLUBILITY RELATIONS OF RHOMBIC SULFUR
Joel H. Hildebrand, Clarence A. Jenks
J. Am. Chem. Soc., 1921, 43 (10), pp 2172–2177
This may contain more sure numbers on sulphur solubility in the relevant solvents.
[Edited on 3-7-2011 by peach]
[Edited on 3-7-2011 by peach]
|
|
|
Natures Natrium
Hazard to Others
  
Posts: 163
Registered: 22-12-2004
Member Is Offline
Mood: No Mood
|
|
Wow, allow me to be the first to congratulate you on an excellent and detailed write-up. As mentioned in this thread I performed this reaction a
while back, but with a significantly poorer lab technique (and equipment).
Ultimately, the only real use that my product was put to was treating some activated carbon for use in the catalytic formation of SO2Cl2. I vaguely
recall that combining SCl2 and SO2Cl2 and refluxing can bring them to an equilibrium of mostly SOCl2, although I honestly cant recall if I have a
reference or it was a flight of hopeful fancy on my part. Will be interesting to see what uses you put it to. 
\"The man who does not read good books has no advantage over the man who cannot read them.\" - Mark Twain (1835-1910)
|
|
|
| Pages:
1
2
3 |
|