Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Diphenylamine via Friedels-Craft-Alkylation?
Good Evening,
Is it possible to add a phenyl-group to an amine?
In this case its about reacting anilline with chlorobenze, to produce Diphenylamine, if necessary with a catalyst (probably needed), like
AlCl3
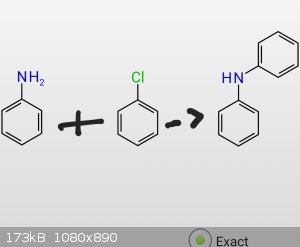
[Edited on 3-9-2018 by Amoled]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
The thing you are looking for is called the Buchwald-Hartwig amination. AlCl3 is not a catalyst for said reaction. Copper, in the Ulmann reaction can
do the same. both of these reactions require fine tuning the ligand, base and solvent to obtain good yields. Low yields are much more easily
achievable.
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Thank you, I will dig into it a little bit deeper, the Buchwald-Hartwig Coupling especially, looks like something I need.
But it looks like you need some complex catalysators for this reaction.
Or better said the Ligands are hard to get for an Amateur chemist
[Edited on 3-9-2018 by Amoled]
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
A variation of the Ulmann reaction is described on prepchem. http://www.prepchem.com/synthesis-of-diphenylamine/
|
|
|
Swinfi2
Hazard to Others
  
Posts: 131
Registered: 19-2-2018
Location: England
Member Is Offline
Mood: Catalytic
|
|
Yeah unfortunately this one doesn't work with the easy method as the product 2° amine is more easily alkylated than the 1°.
I suppose a large excess of the 1° amine and extremely slow/dilute addition -might- work but it's a gamble.
|
|
|
wildfyr
Harmless

Posts: 25
Registered: 23-6-2018
Member Is Offline
|
|
This is a tough one for an amateur chemist. Buchwald-Hartwig amination is the most straightforward, but yeah, it needs Pd and catalysts.
You could try to deprotonate aniline with BuLi, LDA, or NaH, and heat the christ out of it (under pressure) but that isn't very safe or dependable.
Sigmatropic's Ullman reaction is by far the best option.
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Chlorobenzene is not very reactive. I wouldn't expect it to work very well.
Maybe Bromobenzene. http://www.prepchem.com/synthesis-of-diphenylamine/
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
You are partly right - https://pubs.acs.org/doi/10.1021/ol302688u
|
|
|
Amoled
Harmless

Posts: 31
Registered: 2-6-2017
Member Is Offline
Mood: No Mood
|
|
Yeah, this was just a probably bad example, just wanna know if there is a Good way, to benzylate amines - not necessary an amine with a benzene Ring
on it.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Benzylation is different from phenylation (and arylation). With the right nomenclature you're much more likely to find relevant literature. I suggest
using reaxys or scifinder and grouping the reactions into say strong base catalyzed processes or palladium catalyzed, nickel catalyzed, copper
catalyzed ect.
|
|
|