Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Forest Of Amines (Revived) (ALTERNATIVE GABRIEL SYNTHESIS OF AMINES)
Hello Everyone !!
Ther's a Sciencemadness post (http://www.sciencemadness.org/talk/viewthread.php?tid=19483) from 2011 that present's these pathway for the synthesis of Secundary Amines, posible
Primary as well
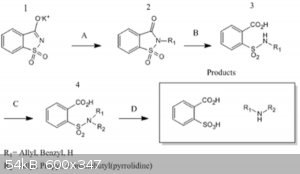
conditions are:
A) Reflux or Heat with appropriate alkylating agent to yield 2.
B) NaOH reflux to 3
C) R-X, then reflux NaOH(aq) to 4
D) HCl(aq), reflux to amines
I've been searching for a project for my Lab, and these seems a very interesting one. But before i go for it, i've wanted to post my beginnings in
case there's someone already doing it, or done it.
Because whell, there's no need to Discover gunpowder twice.
non the less i did some Expeirment's and so far these is what i have done.
In the original post, Chordate has made N-Benzyl Saccharin with Benzyl Bromide, saccharin and potassium carbonate in DMF with a Yield of 28% in 4 - 6
hs. The N-Benzyl saccharin is a known compound and the Melting Point of these is published in the literature (110 - 111 Degrees Celcius) In my amateur
lab i only have acces to these for the characterization of the produt, i will however send some product for NMR and IR spectoscopy, the IR is more
accesible, in the lab i work at University ther's one i can use. But it will take me TIme, Here in Argentina 80 Dolars is hard to get to pay the
NMR.DMF is difficult to get for the amateur, and Though i have some i rather not use it, so i tried different solvents and use the Melting point to
see the purity and characterize my compound. The reaction i'd tried was Reacting Benzyl chloride and Sodium Saccharin (10ml with 15gm in 25ml of more
of solvent) Then isolation adding water and recrystallization with Ethanol 96%
Acetonitrile at 10hs Reflux (Bp 81 degrees) No product (even with KI catalyst )
Dioxane at 10hs Reflux (Bp 101 degrees) No product
Dioxane/DMF (2:1) 10hs Reflux ... No product
Butyl Acetate 10hs (Bp 126 degrees) with KI catalyst No product
Di-butyl phthalate 10hs heat at 160 degrees (Bp 360 degrees) No product
Butyl-Glycol 10hs (Bp 171 degrees) 17,8% Yield (Yay First Product)
Ethylene Glycol 2 1/2 hs (Bp 195 degrees) 25,5% Yield
Okay, so Ethylene Glycol works and yield is just under what Chordate
got ( But without DMF ) Okay then i tried using Catalyst, conditions where the same (10ml benzyl chloride 15g Na-Saccharin, 25ml solvent and 0,5g
catalist at reflux 2 1/2 hs) Isolation with water and recristaluzation with ethanol 96% (all using the same amounts, as best as i cud, to compare
results)
Using KI ... Yield was 6,22g (23,4%) Mp 110-112
Using KBr ... Yield was 6,25g (23,5%) Mp 108-110
Without catalyst ... Yield was 6,8g (25,5%) Mp 103-110
I believe that catalyst desn't work in these protic polar solvent (It was expected ) but i gave it a try anyway.At the moment i don't have TLC plates
(Super expensive here in Argentina. SUPER EXPENSIVE  ) But im going to buy theme
only if these research is Useful. At least there's an Improvised method with an Alternative solvent for DMF at almoust the same Yield. so far i used
Benzyl chloride because it has a High boiling point, and is reactive and easy to use. I have other alkyl halides to try but before i use theme. The
Benzyl one was more simple and easy to do ) But im going to buy theme
only if these research is Useful. At least there's an Improvised method with an Alternative solvent for DMF at almoust the same Yield. so far i used
Benzyl chloride because it has a High boiling point, and is reactive and easy to use. I have other alkyl halides to try but before i use theme. The
Benzyl one was more simple and easy to do
okay so far these is what i have done. I hope these is useful, and looking forward to the coment's to see if you think i should continue. I leve here
Papers that i used as reference. have a nice day.. Your'e Argentin fellow Chemist
https://pubs.acs.org/doi/abs/10.1021/ja01870a004
https://pubs.acs.org/doi/abs/10.1021/ja01630a099
[Edited on 2-8-2018 by Chem Science]
[Edited on 2-8-2018 by Chem Science]
[Edited on 2-8-2018 by Chem Science]
[Edited on 2-8-2018 by Chem Science]
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
SOME NOTES FROM EXPERIMENTS
BUTYL-GLYCOL REACTION
On a reflux equipment i reacted 15g of Na-Saccharin, 10ml of Benzyl Chloride, and 50ml of Butyl Glycol. After Reflux i add 200ml of water and it
separated an oil and a precipitate. These was filterd and the precipited recristalized in Ethanol 96%. Yield 4,73g or 17,8% Mp 110-112
The oil wass wash with water and dry with CaCl2 (It actualy seems to be a mixture of thing's, it disolves some CaCl2 and mix with water a little bit
... yea i have no idea) I Fractionaly distill it and these came over.
Fraction 1 around 10 ml of milky liquid around 105 - 110 degrees. Smells like butanol
Fraction 2 around 10ml of clear liquid at 110 - 120 degrees
Fraction 3 A little more than 10ml of clear liquid at 160 - 166 degrees.
The last fraction was mix with water (washed) and some dissolved, the rest was washed again and dried with Molecular sieves and saved for NMR analisis
and GC-MS
all of the fractions are reactive to acidic Dichromate, the last one being more reactive.
In the Ethylene Glycol reactions very little oil was obtained.
[Edited on 2-8-2018 by Chem Science]
[Edited on 2-8-2018 by Chem Science]
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2800
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
Saccharin is difficult to obtain in quantity; if you can buy bulk saccharin, you can probably buy phthalimide. I learned this when considering
N-halosaccharins as oxidants.
Sulfonamides are generally harder to cleave than carboxamides, alkali not preferred in this case. N-deprotonation prevents nucleophilic attack by
hydroxide. HBr in acetic acid with bromine scavengers is a system of choice.
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
Hi clearly_not_atara 
Well, actually Argentina is a Rather Wird place, Here Saccharin is easier to obtain, Phthalimide is very hard, The problem with the Phthalimide
approach, is the use of Hydrazine. I cant get these easy. The hope of these Reserch is to see how hard it is to make it to work at Amateur level. I
did had an idea of the Resistance on hydrolisis, i hope to use these as an Advantage. O well, seems these is a -1 point.
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Hi Chem Science were obtain Benzylchloride in Pampaland?
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
GarayGhost ..You have an Amateur lab ? In Argentina ? We should talk then. Chem.Science@outlook.com
I actually synthesize my Benzyl Chloride From Benzyl alcohol that i buy from Cicarelli ( Córdoba) (Somewhat expensive) Reflux 500ml HCl and 100ml of
Alcohol for 1h, separate phases, dry with CaCl2 and Distil.
[Edited on 3-8-2018 by Chem Science]
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chem Science  | GarayGhost ..You have an Amateur lab ? In Argentina ? We should talk then. Chem.Science@outlook.com
I actually synthesize my Benzyl Chloride From Benzyl alcohol that i buy from Cicarelli ( Córdoba) (Somewhat expensive) Reflux 500ml HCl and 100ml of
Alcohol for 1h, separate phases, dry with CaCl2 and Distil.
[Edited on 3-8-2018 by Chem Science] |
Benzyl alcohol is otc? Im live in city of Cordoba province.
|
|
|
Chem Science
Hazard to Others
  
Posts: 123
Registered: 30-7-2018
Location: Argentina
Member Is Offline
|
|
We can Speak spanish you know xD jajajaja
La empresa TodoDroga (Ahora se llama PuraQuimica) Trabaja productos Cicarelli ( https://www.tododroga.com.ar/laboratorio/ ), y allí yo les compre Alcohol bencilico sin problemas, es medio costoso, ami me salio algo así como
500 y algo pesos el litro en ese tiempo ( hará 1 año ). Pero se consigue.
|
|
|