boilingstone2
Harmless

Posts: 6
Registered: 6-7-2017
Member Is Offline
Mood: No Mood
|
|
Synthesis of Fomepizole
Hi all,
I have a friend that suffers from a severe case of "asian glow", to the point where even a few sips of beer will put them out of commission with a
flushed face, headache, and nausea. While I would never consider administering anything I might make to this person, it got me thinking about possible
solutions to my friend's problem. AFAIK, "asian glow" is caused by a buildup of acetaldehyde in the body, due to a more active version of the alcohol
dehydrogenase enzyme that some asian people possess. Inhibiting the alcohol dehydrogenase enzyme should reduce the rate at which acetaldehyde is
produced, allowing the body to process the acetaldehyde as it is formed, solving the problem.
An example of an alcohol dehydrogenase inhibitor already used in medicine is Fomepizole, or 4-methylpyrazole. An example synthesis is shown below:
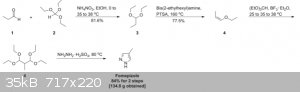
This route doesn't seem very OTC, so I wanted to hear your thoughts on an alternate synthesis pathway I've been trying to come up with.
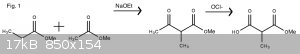
To start, one would first synthesize methylmalonic acid by performing a Claisen Condensation with methyl acetate and methyl propionate to form the
beta-ketoester, methyl acetopropionate. Then using hypochlorite, one should be able to perform a haloform reaction to reach a methylmalonic acid (Fig.
1).
By condensing methylmalonic acid with hydrazine acidified with sulfuric acid, one should arrive at a pyrazole derivative, in a similar way to the
reaction shown below:

Finally, assuming it is similar enough, one would perform a reduction like the one described here for barbituric acid to arrive at Fomepizole:
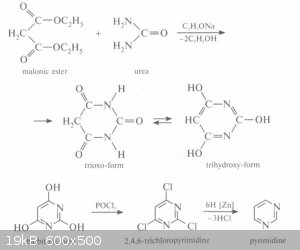
I feel like it's a little thrown together, but do you guys think I'm on the right track with this?
I guess one of my questions is, can you perform a condensation with methylmalonic acid and hydrazine, or does the methylmalonic acid have to be an
ester first?
While this might be more amateur friendly, (no BF3), I see it still uses POCl3, which is still a problem.
|
|
|
DavidJR
National Hazard
   
Posts: 908
Registered: 1-1-2018
Location: Scotland
Member Is Offline
Mood: Tired
|
|
An apparently well-known solution to this problem is an OTC medication called ranitidine (brand name Zantac + others). It's a histamine 2 receptor
antagonist, used to suppress stomach acid production. However I know from experience that it definitely potentiates the effects of alcohol, presumably
by inhibition of alcohol dehydrogenase.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Another OTC route
1.2-methyl-1,3-propanediol from formaldehyde -https://onlinelibrary.wiley.com/doi/abs/10.1002/cber.1942075...
2.bleach oxidation to 2-methylpropanedial
3.Fomepizole -http://www.orgsyn.org/demo.aspx?prep=CV4P0351
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
I've posted about a patent covering an OTC route to pyrazoles before. http://www.sciencemadness.org/talk/viewthread.php?tid=76527
It involves the slow addition of proprionaldehyde in (m)ethyl formate to a solution of sodium (m)ethoxide in (m)ethyl formate. To give the sodium salt
of methyl malondialdehyde. Which upon treatment with hydrazine (sulfate) gives fomepizole. Whether or not one has to do an intermediate isolation of
methyl malondialdehyde in some form or another is unknown but I would bet fomepizole can be vacuum distilled or crystallized.
There are several other approaches mentioned in the thread not the least of which is starting with an appropriately substituted acetylacetone,
condensation to the pyrazoles, oxidation of the methyl groups to Carboxylic acids followed by dry distillation to achieve decarboxylation.
Time for someone with time and a lab to get around to trying some of these routes.
Edit2:
Forgot that the 4-methyl of fomepizole is bound to be oxidized as well. Perhaps the route mentioned by atara is applicable. The diazoacetate should be
accessible from alanine ethyl Ester. Could be as simple as diazotization of alanine, cycloaddition with acetylene, saponification and decarboxylation.
Good luck experimenting!
[Edited on 28-5-2018 by Sigmatropic]
[Edited on 28-5-2018 by Sigmatropic]
|
|
|
Waffles SS
Fighter
   
Posts: 998
Registered: 7-12-2009
Member Is Offline
|
|
All possible way for synthesis Fomepizole by Reaxys
Attachment: Fomepizole.pdf (54kB)
This file has been downloaded 1006 times
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
The structure of one of your examples is very similar to that of Cyanuric Acid.
https://en.wikipedia.org/wiki/Cyanuric_acid
In the case of Cyanuric Acid, chlorination might not proceed in the desired manner. Halogen atoms may bond directly to N.
https://en.wikipedia.org/wiki/Trichloroisocyanuric_acid
[Edited on 16-6-2018 by zed]
[Edited on 16-6-2018 by zed]
|
|
|
unionised
International Hazard
    
Posts: 5126
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Concurrent use with ethanol is contraindicated because fomepizole is known to..."
From wiki
https://en.wikipedia.org/wiki/Fomepizole
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
A thousand dollars per vial? How much is in a vial? I like the sound of the economics.
|
|
|