Vanry
Harmless

Posts: 36
Registered: 13-12-2017
Member Is Offline
Mood: covalent
|
|
Synthesis of phenol from benzene
Hi everyone ! I've been checking around to answer this question but can't seem to find anything reliable or detailed enough to satisfy me. It feels
like "oh just put this and this and TADAAA"
I got the impression that it is too well known to have any documentation so I can't find any 
so anyway:
Is there a viable way to synthesize phenol from benzene in lab ?
(In fact it is part of a full process from sodium benzoate to catechol, so if you got better than:
benzoate -> benzene -> phenol -> catechol, knowing that i already know the first and last part, don’t hesitate to contribute and annihilate
all my search so far if you got a shorter route  ) )
ok so what i found so far:
option1: through chlorination
Benzene + Cl2 -> chlorobenzene
chlorobenzene + NaOH -> sodium phenoxide ---acidify --> Phenol.
advantages:
- chlorine react strongly, so I would expect hight yield.
- It exist. Sorry but I really need more infos to find more advantages, so it's an advantage 
problems:
- no idea of the real yield
- no idea of temperature conditions, time of reaction, solvent (if any), catalyst
- selectivity ? why chlorobenzene would be produced and not dichlorobenzene or hexachlorobenzene ? how to separate them then ?
- CHLORINE for christ’s sake ! It's a strong disadvantage, especially since it seems impossible to get a decent gas mask in france... But If I
don’t have any other choices, I’ll do it this way.
option 2: through sulfonation
Benzene + Oleum (H2SO4 + SO3) -> benzenesulfonic acid
benzenesulfonic acid + NaOH --200°C-> sodium phenoxide ---acidify--> Phenol.
advantages:
- not as dangerous as the first one (will still dissolve the ground, but not my lungs)
- seems selective to me (at least more than the Cl2 above and the H2O2 methods bellow)
problems:
- reactant. Oleum is pretty hard to find/made at home.
then pretty much the same than before:
- no idea of supposed yield
- no idea of precise selectivity
- rarity and dangerosity of reactant
Option 3: direct oxidation with peroxyde
benzene + H2O2 ---catalist--> phenol
advantage:
- won’t kill me
- can be selective and high yields with correct procedure reagent and catalyser
problems:
- i need a procedure that is clear and the good catalyser, because hydrogen peroxyde is not really a selective reactant.
Option 4: cumene process
i don’t have industrial supply to play with, but mentionned this so no one will go on this way ^^
okay finally a question recap:
Does somebody know how to produce phenol from benzene on a lab scale ? Does anybody can clarify or add something to one of the previous methods ?
thanks for reading so far, all my excuses for my english as I'm not a native english and thanks in advance for all youre answers ! 
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
1. Nitrate the benzene with HNO3/H2SO4.
2. Convert the nitrobenzene to aniline via Sn/HCl
3. diazotize the aniline with NaNO2/HCl at 0 °C
4. convert the diazonium ion to phenol using water/H+.
[Edited on 26-4-2018 by Magpie]
[Edited on 26-4-2018 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
That's the first procedure that came to mind for me too, Magpie, though it's certainly going around your elbow to get to your ass.
Vanry: There are some other issues with the procedures that you've suggested. The chlorobenzene route is problematic because the chlorination isn't
selective. You will get s mixture of chlorobenzene and various polychlorinated benzenes. Furthermore, that hydrolysis is a lot more difficult that
you'd expect. Just look up threads where people try to make hydroquinone from p-DCB.
As for the sulfonic acid route, i haven't seen anyone actually do that, but the reaction with sodium hydroxide involves actually fusing the
benzenesfonate with molten sodium hydroxide. It's not fun and it's definitely going to produce a lot of charred impurities.
Peroxide is sketchy and unselective and I'm not sure such a route actually exists. From quick searching all I could find were industrial gas phase
routes that are out of the question.
Though I assume you've probably already cast it aside for some reason, it would be much, much easier to simply make phenol from salicylic acid by
decarboxylation. Otherwise, the method that Magpie outlined is the only proven method that I can think of despite it being quite tedious for making
something so simple.
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
The nucleophilic substitution of chlorine on chlorobenzene is extremely slow. This is because an SN2 mechanism is stereoelectronically
prohibited (C-Cl σ* orbital is "buried" inside the ring); SN1 will be slow as a result of Cl- not being a sufficiently-good
leaving group (which also prevents an SRN1 process) and the concomitant loss of aromaticity.
Have you considered trying something similar to the industrial cumene process? Benzene is alkylated to cumene (on a lab scale this could be achieved
with 2-bromopropane under Friedel-Crafts conditions (or 1-bromo with a good Lewis acid). Cumene is then oxidised to cumene hydroperoxide (normally
with high-pressure O2 - but perhaps this step could be done otherwise), which can then undergo something resembling a Baeyer-Villiger
rearrangement to afford phenol and acetone.
I wonder (this is purely speculative) if you could start with 2,2-dibromopropane? This gives scope to do the F/C alkylation (loss of the first
bromide) and then generate the required benzylic carbocation by loss of the second bromide. This could then be treated with basic hydrogen peroxide to
give cumene hydroperoxide.
I'm not sure whether the F/C alkylation can be done with the gem-dihalide (the remaining halogen strongly destabilises the carbocation). This effect
might even be strong enough to favour the primary carbocation over the secondary position in this case - thus giving you the wrong connectivity to the
aromatic ring. Alternatively, the carbocation may not form at all.
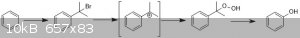
[Edited on 26-4-2018 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
walruslover69
Hazard to Others
  
Posts: 235
Registered: 21-12-2017
Member Is Offline
Mood: No Mood
|
|
I feel like you might be able to get somewhat reasonable yield sulfonating by refluxing with excess 98% sulfuric acid. If not you can produce and
bubble SO3 through sulfuric acid by the pyrolysis of sodium bisulfate. When carrying out the alkali fusion keeping your vessel air tight at those
temperatures is going to be essential.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Chlorination of benzene is a viable route but requires the use of anhydrous FeCl3 or AlCl3 as a Lewis acid catalyst to induce a dipole in order for it
to react. Bromine, as per this example, would be better due to the higher electron density hence would be a faster reaction - the aforementioned
catalysts can still be used despite not being bromides.
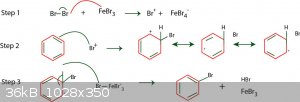
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
It's true that the bromination is more controllable than chlorination, I've done it before with bromine and a trace of aluminum foil to generate the
catalyst in situ.
However, the SN1 hydrolysis, although theoretically faster with bromobenzene than chlorobenzene, is still very unfavorable, as Hexavalent
explained. Have you actually tried it, O Learned One?
[Edited on 4-27-2018 by Texium (zts16)]
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Admittedly, no, since I don’t have access to benzene or bromine. I was just going off the theoretical chemistry I learned at school, since we
covered some substitution reactions to benzene (there was a whole topic on benzene for OC in our A2 level course) and this was one of the main ones,
as well as FC alkylation amongst others.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
I was referring to the hydrolysis since that's the sticking point here, if you'd read closely. Bromination of benzene is easy peasy.
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Oh, my bad for not paying close attention; I’ve done hydrolysis of haloalkanes in school to compare the rates between -Cl, -Br, and -I but not
halobenzenes.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Vanry
Harmless

Posts: 36
Registered: 13-12-2017
Member Is Offline
Mood: covalent
|
|
first: thank to all for answering so fast !
Quote: Originally posted by Magpie  | 1. Nitrate the benzene with HNO3/H2SO4.
2. Convert the nitrobenzene to aniline via Sn/HCl
3. diazotize the aniline with NaNO2/HCl at 0 °C
4. convert the diazonium ion to phenol using water/H+.
[Edited on 26-4-2018 by Magpie]
[Edited on 26-4-2018 by Magpie] |
very appealing, but has 2 major drawback: it involve lot of reactions (hight cost in time and reactant) and the second one is NaNO2. You really think
a country that prohibit gasmask (I won't ever stop complaining about the stupidity of this...) will allow its citizen to freely buy some strong
oxydizer ? Yup; I can't. maybe it can be produced but it add another step. But well, more viable way I get so far, so I will look around for NaNO2 and
keep that in mind ^^
for the benzenesulfonate way:
@Texium: don't worry for the method of melting NaOH, I already apply it to get my benzene from the sodium benzoate and it works pretty well. not sure
if I can get rid of all impurities you're talking about by distillation, but still, it is viable.
@walruslover69: Yup I was just reading something about that, It seems possible to sulfonate just with concentrated sulfuric acid, and Thx for the SO3
production through pyrolysis idea, I will definitely try out.
for the chlorine/bromine way:
first of all with safety: bromine is worse than chlorine for me in my lab. Because I got a strong ventilation and air filtering system, but it is
almost useless against a spill and one big disadvantage of my lab is that the ground is really difficult to clean as it is under the surrounding
ground level and a part of it is not flat (sorry guys I’m doing what I can. But you’ll understand  ). ).
I wasn't really confident into this way since I started, and I'm still really not. I think the presence of a catalyst will dramatically increase
yield, and bromine is more selective. But as aforementioned, it get stuck on hydrolysis wich is a bit tricky.
Cumene process and derivative:
it involve lot of reaction and are generally adapted to industrial level. It may be possible to downsize it to lab level, but it involve a stupid
amount of work and money, where the goal here is to reduce this cost.
from salicylic acid
yup, I read about that on another thread, but here I wan't to take advantage of my almost free and almost limitless supply of benzene, and salicylic
acid is not cheap and not abundant at all. unless you can give me some reliable delivering sources, that can deliver in Europe, I think it’s better
not to go on this way. but, hey, I may be surprised 
from hydrogen peroxyde
I was wondering something. As the final goal here is catechol, isn’t it more simple to just overshoot like:
benzene + H2O2 ---> phenol + catechol + hydroquinone
And then, finish it with my method to transform Phenol in catechol to get what I want. The only problem lasting is: is there any possible way that it
react differently ? do you think hydrogen peroxyde will react at all and/or form random things ?
thanks again for answering ^^
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Nitrates are oxidising agents, nitrites are reducing agents. If possible, you can buy NaNO2 as a meat curer, and if you live within the EU, you should
be able to easily order it from the UK - I’ve never had any trouble purchasing it in pure form (>99% food grade).
If you do manage to get your hands on some NaNO2, then be careful because it will react with most acids to produce clouds of NO2 which is highly
poisonous and smells really strongly like sharp bleach. When adding your acid to the NaNO2, do it dropwise and cool it down to below 10 degrees C,
preferably between 0 and 5 to avoid releasing clouds of red-brown NO2 if your solutions are concentrated; I’ve done this countless times at room
temperature using dilute solutions of nitrite and acid with whatever I’m trying to react, mainly making nitrite esters and performing Sandmeyer
reactions.
With the latter, which is what you’re doing here, I’d actually advise you to keep it at room temperature since this promotes a quick reaction of
the diazonium intermediate with water which manifests as lots of tiny bubbles of nitrogen gas. Just keep the addition really slow so that there never
gets more than a slight orange tint in the flask - it will disappear as it is displaced by nitrogen gas but isn’t enough to harm you, keep the
ventilation up anyway though even if there is no smell. Add your acid solution at no more than 2-3 drops every 5 seconds until you are near the end of
the reaction, then you can dump the rest in faster and faster since the concentration of NaNO2 will always be decreasing, using the colour of the
flask as a quick visual indicator. Whenever I’ve done this, I use H2SO4 and the concentration is never above 4M (1mL 96% H2SO4 topped up to 5mL with
water).
Sorry for dragging this point out but I can’t stress how incredibly useful NaNO2 is in organic chemistry, when it comes to forming diazonium
intermediates which react with so many chemicals, both organic and inorganic. Nitrite esters are also useful protecting groups, but this isn’t
entirely relevant here.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
Sigmatropic
Hazard to Others
  
Posts: 307
Registered: 29-1-2017
Member Is Offline
Mood: No Mood
|
|
Halo benzenes are not substituted by an Sn1 mechanism. Nor is an empty sp2 orbital sticking out of a phenyl a real thing. Not even in sandmeyer type
reactions.
Depending on electronic factors aromatics are substituted by the two flavors of nucleophilic aromatic substitution. Either the two step
addition-elimination with the intermittent meisenheimer complex or the elimination to benzyne followed by trapping with the nucleophile.
Chlorobenzene is bound to react via benzyne. Which is also the reason why dichlorobenzenes (ortho, meta, para) form resorcinol on fusing with alkali.
One other approach I can think of is to make a halobenzene, then form the grignard and add it to a boron compound of choice (say trimethoxy borate).
The boronate can then be hydrolyzed with H2O2 and base. Anyway it is a lot of work for such a simple compound.
|
|
|
Vanry
Harmless

Posts: 36
Registered: 13-12-2017
Member Is Offline
Mood: covalent
|
|
Quote: Originally posted by LearnedAmateur  | Nitrates are oxidising agents, nitrites are reducing agents. If possible, you can buy NaNO2 as a meat curer, and if you live within the EU, you should
be able to easily order it from the UK - I’ve never had any trouble purchasing it in pure form (>99% food grade).
If you do manage to get your hands on some NaNO2, then be careful because it will react with most acids to produce clouds of NO2 which is highly
poisonous and smells really strongly like sharp bleach. When adding your acid to the NaNO2, do it dropwise and cool it down to below 10 degrees C,
preferably between 0 and 5 to avoid releasing clouds of red-brown NO2 if your solutions are concentrated; I’ve done this countless times at room
temperature using dilute solutions of nitrite and acid with whatever I’m trying to react, mainly making nitrite esters and performing Sandmeyer
reactions.
|
My bad, I used à poorly traduced word. “Oxydizer” I don’t know the English for “help to burn” like oxygen. I’m thinking you don’t have
a word for that in English but don’t matter because it’s the same.
Anyway, failed on the chemical too so it doesn’t matter at all x)
Available for me yes ! And not too pricy, so I will definitively give it a try ! Thanks  I’ll try this and my overshoot method with H2O2 and try to find what is best I’ll try this and my overshoot method with H2O2 and try to find what is best 
|
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
No, you didn't mistranslate, oxidizer or oxidizing agent, same thing. And sodium nitrite can be an oxidizer in some cases, while it acts as a reducing
agent in others. However, it is never a strong oxidizer as you said it was. It is a weak oxidizer at best, and that property would not be a reason for
it to be regulated.
I still think that you'd be better off making phenol from salicylic acid, which in turn can be made easily from acetylsalicylic acid (aspirin). Even
if the raw materials cost more (which they probably won't, anyway), you save so much in time and labor that it is definitely worth it. Save your
benzene for other projects.
[Edited on 4-27-2018 by Texium (zts16)]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
In the U.S., Benzene is hard to come by. Expensive, and because of its toxicity, scarce.
Last time I checked, its cost was about 100 U.S. Dollars per U.S. gallon. (8 pounds)
Lots of environmental regulations apply.
Cheap... ( only a few dollars a gallon) if you buy it by the ton... Provided, you can find someone to sell it to you.
Acetyl Salicylic Acid might be a better starting material. It is relatively inexpensive, and readily available.
https://www.healthypets.com/aspow1pounby.html?mr:trackingCod...
Note: I don't know where in the world you are, but in my jurisdiction, certain folks have been arrested for acquiring Phenol. It is a precursor to a
TNT type of material.
Are you really harmless, Vanry?
[Edited on 27-4-2018 by zed]
[Edited on 27-4-2018 by zed]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
OK firstly disregard any possibility of SN2-like reactions. Only a SNAr would work and even then, you'd need a specifically prepared aromatic ring
with adequate substituents. Maybe 2- or 4-chlorosulfonic acid could even work (and then removing the sulfonic acid is trivial).
Anyway, if SNAr is not available for you -as you've mentioned that diazotisation is beyond your scope-, the only other drastic possibility I can think
about is something similar to the Buchwald-Hartwig amination but with an alcohol. With subsequent cleavage of the ether...somehow.
Things: even if you can't find Pd, or it is too expensive for this use (which fair enough), try nickel and even iron salts. I recently read a paper
where 95+% yields were achieved in a Negishi coupling with iron acetate (or another salt, I can't remember). So you may as well give it a go.
And for your interest, read about the reactivity of aromatic systems, it is extremely different from that of alkyl halides.
[Edited on 28-4-2018 by Eddygp]
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Vanry
Harmless

Posts: 36
Registered: 13-12-2017
Member Is Offline
Mood: covalent
|
|
@zed: is you’re final question ironical ? I’m not sure to understand right :/ If you were serious: I’m no dealer or terrorist I’m juste a
curious chemistry student ^^
And btw: I’m from France, the country were chemical law gives no damn sense, that’s why you are probably intrigued. I spend the last 5H searching
this mess and I found out: I’m not allowed to buy a gas masks BUT I can tell the cops to fuck themselves if I got under 100L of any product (they
call it « low quantity »)
God, can you imagine that ? I can take up to 3 YEAR of jail for a gas mask and 2 filters but 99L of benzene and 80L of bromine ? Totally right for the
law. Still: benzene selling is prohibited, but detention is not (go ask them why). And this is the same for every product. Only chemical war
agent/poison (poison = goal is to kill and nothing else, benzene kills but is a reactant), explosives, psychotropes and their precursors are
prohibited to possession. Phenol and benzene are not part of this list (in France at least) but they are forbidden to selling to particular.
I might check out aspirin seller for animals, I don’t thinked of it thanks 
@Eddygp: as you guessed, I am really poorly documented on aromatic reactivity. Time to read more  Btw if you got books to recommand on the subject don’t hesitate Btw if you got books to recommand on the subject don’t hesitate 
But what you send about diazotization being out of my scope, I just was wondering if there was any simplest way. And now I just checked in more detail
with the supplier and it is beyond my scope as NaNO2 is forbidden to selling to individual in France. Yes oxidizer as I guessed.
And as you said, the need for precisely substitued aromatic ring add even more complexity to this mess. Not sure at all to try this. I know it start
to get out of my reach when you write « removing sulfonic acid is trivial » and I don’t get any idea on how to do so. But hey, every travel has
a starting point. can you explain more about this trivial method ? ^^
As I see things now:
- document on aromatic reactivity
- try benzene + H2O2 random-beginner-luck-way
- search for aspirin method and supplier and keep my benzene for something else
- search unofficial supplier for NaNO2
Thanks all so far for youre contributions 
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
Systematic Organic Chemistry has directions for chlorobenzene, benzenesulfonate, and phenol. Oleum is not needed, but the NaOH heating goes up to 330C
there not 200.
|
|
|