metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
How to trigger reaction in closed vacuum jar ?
I want to test reactions under vacuum.
I use for it an 1.2 liter glass 'weck' jar which can easily be evacuated and want to use KMnO4 + glycerin to trigger further reaction.
But because I have no access to the contents of the jar when it is closed for evacuation, I can not drop the glycerin on the KMnO4.
I tried the glycerin on a piece of paper and shaking after evacuation, but the wetted paper did not reach the KMnO4 pile.
Does someone have a 'trick' to mix these chemicals without having access to the content of the jar ?
|
|
|
OldNubbins
Hazard to Others
  
Posts: 136
Registered: 2-2-2017
Location: CA
Member Is Offline
Mood: Comfortably Numb
|
|
magnets?
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Freeze the glycerine, then let it melt once the vacuum's pulled?
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
VSEPR_VOID
National Hazard
   
Posts: 719
Registered: 1-9-2017
Member Is Offline
Mood: Fullerenes
|
|
A 500mw laser
https://www.amazon.com/Adjustable-Focusable-Printer-Engravin...
Within cells interlinked
Within cells interlinked
Within cells interlinked
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Just let the glycerin in through the vacuum port without letting in any air. Be aware though, glycerin is extremely viscous and might take forever to
get through a long, small opening.
Technically you should de-gas the glycerin as well, if that matters for your rxn.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2757
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
If you just squirt the glycerine on the KMnO4, then close it up, it usually takes a minute or so to start, would that be long enough to evaluate the
jar? Or put the glycerine in a small upright beaker, vial or test tube, and tilt it to pour it.
|
|
|
j_sum1
Administrator
       
Posts: 6336
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Cody of Cody's Lab fame has been doing quite a bit recently using a vacuum chamber. I have seem him employ quite a few different methods for
manipylating objects in the container under vacuum.
Magnets is the obvious choice here. Suspend an object using a magnet or piece of iron on the inside and a magnet outside. Remove the outside magnet
and let it drop.
Electromagnet - then you can flick a switch.
Rig up something using a remote control. You'll need a power supply and receiver inside the vacuum though.
Go for something clockwork - a wind up toy or alarm clock or something.
Suspend on a thread and burn with a laser.
Variations can drop an object directly or drop a mass to manipulate something indirectly using levers or pulleys or simple linkages.
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
Inject the reactants through a suba seal septa?
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
Harristotle
Hazard to Others
  
Posts: 138
Registered: 30-10-2011
Location: Tinkerville
Member Is Offline
Mood: I tink therefore I am
|
|
See diagram.
Basically use glass bottle with septum made from icecream lid. Tilt to mix. Tape to side of vacuum jar.
Adjust material for compatibility with whatever you are studying.
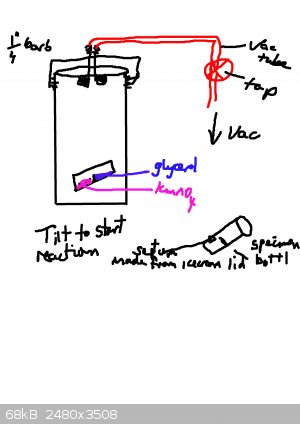
Cheers,
H.
|
|
|
Sulaiman
International Hazard
    
Posts: 3728
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I can imagine so many uses for electrical connections to a vacuum chamber,
automotive spark plugs or 4mm binding posts with lots of epoxy ?
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Σldritch
Hazard to Others
  
Posts: 310
Registered: 22-3-2016
Member Is Offline
Mood: No Mood
|
|
Maybe i Harristotle already said this but i had a hard time understanding it...
Put something like a balloon under the your reaction pot and let Glycerine flow when it is tilted by the expanding balloon. That is of course if you
can not do it with magnets or lasers which seem much more convenient and reliable.
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
Am I missing something? Why can't it simply be injected into the system through a seal?
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
I have a laser, but that only ignites KClO3 + sugar in air only and even then hardly, so too faint.
| Quote: | | Why can't it simply be injected into the system through a seal? |
Well the 'seal' won't work very well when the jar is drawn vacuum.
I'll give the tilting metal a try.
|
|
|
DrP
National Hazard
   
Posts: 625
Registered: 28-9-2005
Member Is Offline
Mood: exothermic
|
|
I think I had the same problem once... I cut up and stuck together a few suba seals and made a double or triple septum.
\"It\'s a man\'s obligation to stick his boneration in a women\'s separation; this sort of penetration will increase the population of the younger
generation\" - Eric Cartman
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
When the reaction starts and the bottom of the glass container gets suddenly very hot, it will shatter.
At that point you won't be working in a vacuum (you may be looking for the ER instead)
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Here is my setup.
Photo 1: the complete setup, 2: the jar only, 3: another lid for the jar with electrical wires into the vacuum chamber for electric ignition, hose
barb and pressure gauge should be installed in other two holes. Might be easier than the KMnO4 + glycerin tilting option.
In photo 2 the reactants are put in a cell concrete vessel to prevent suddenly overheating the glass jar.
  
[Edited on 2018-3-17 by metalresearcher]
|
|
|
NEMO-Chemistry
International Hazard
    
Posts: 1559
Registered: 29-5-2016
Location: UK
Member Is Offline
Mood: No Mood
|
|
Do medicine capsules dissolve in glycerine? I have never tried, but those huge 500mg ones emptied then filled with glycerine. As it melts its way
through the capsule onto the KMnO4. Or dont fill it all the way, as you pull a vac would the capsule separate?
No idea if any of it would work, but you see my line of thinking? maybe you could come up with something along those lines.
Or make gelatin capsules and fill them, as you pull a vac they melt as the melting point drops. Might need to warm the chamber slightly
Or go hitech, arduino with bluetooth shield and a model servo, have a vial with the goo attached to the servo and pour it on via the blue tooth
commands......
[Edited on 18-3-2018 by NEMO-Chemistry]
[Edited on 18-3-2018 by NEMO-Chemistry]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
I have not read this thread thoroughly so please for give me if someone has already posted this but some reactions can be triggered with UV light.
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
metalresearcher
National Hazard
   
Posts: 758
Registered: 7-9-2010
Member Is Offline
Mood: Reactive
|
|
Finally I got it, but now with electric wires through a sealed hole in the lid.
I used KClO3 + sugar in a small vial and put a Kanthal wire coil on it and after evacuating the jar I chased a current of 8 amps through it. There was
almost no sound, unlike in open air when it gives a strong sizzling sound.
https://www.metallab.net/jwplayer/video.php?v=L2NsaXBzL0tDbE...
|
|
|