Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Best method to make potassium dichromate from stainless steel
After a lot of research I found out this .
Stainless steel contains about 15 to 18% Chromium and rest is iron with little carbon and nickel content.
I dissolved it in HCl and left it for 24 hrs then I precipitated all the iron and chromium carbonates by adding calculated amount of sodium carbonate.
Filtered it,heated it to dry and convert in into oxides and then mixed it with KNO3 and Na2CO3( calculated amount) again heated it , dissolved it in
hot water got a yellow filtrate then I added calculated amount of NH4Cl to decompose all the nitrite formed. Boiled it and concentrated it . Add
calculated amount of after cooling HCl and got orange crystals of K2Cr2O7. Purity of K2Cr2O7 can be tested by doing Chromyl chloride test. This
K2Cr2O7 is free from sulphate impurity but may have Chloride impurity for that it has to be washed with ice cold water.
Today I posted a video on this at my channel if you are interested you may visit and see . I am not posting any links here from now onwards because it
is against Youtube policy.
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
K2Cr2O7 can be quite easily further purified. It crystallizes very well and its solubility curve as function of temperature also is a pleasant one.
Dissolve the material in as little as possible of hot water and then slowly allow to cool (e.g. wrap the bottle with liquid in a towel so that it
cools down slowly). When cooled down to room temperature, put it in a fridge to further cool down. Then decant the liquid from the crystals, press/dry
them in filter paper (not in tissue paper!!) and let them dry in air at a place free of dust.
In this way you get very pure K2Cr2O7. The liquid also contains still some K2Cr2O7. You can recover that as well by boiling the liquid down and then
allowing that to cool down, but this crop of crystals is less pure. I doubt whether it is worth the effort to try to isolate this last little (less
pure) amount.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
I can't find solubility curve of potassium dichromate on internet can you provide me with that
|
|
|
woelen
Super Administrator
        
Posts: 8027
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I don't have such a curve, but from experience I know that it dissolves much better in hot water than in cold water and that it crystallizes very
well. K2Cr2O7 really is one of the chemicals which is very pleasant when it comes to purification by means of recrystallizing.
Just be careful with the stuff, it is a known carcinogen and some people can have strong allergic reactions on exposure.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Here's one i just made from https://en.wikipedia.org/wiki/Solubility_table
(the 70C figure is interpolated because it was missing)
Still not found how to make great graphs in LibreOffice - yet . Gimme a sec.
Data :
0 4.7
10 7
20 12.3
30 18.1
40 26.3
50 34
60 45.6
70
80 73
[Edited on 15-3-2018 by aga]
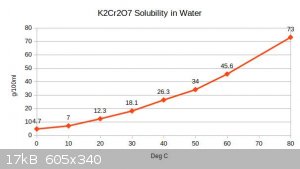
(whoops. had the axis labels back-to-front)
[Edited on 15-3-2018 by aga]
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Wikipedia says that solubility of K2Cr2O7 at 0℃ is 3.6g/100ml water and pubchem says 4.9g/100ml water at℃ which one is correct
|
|
|
Sulaiman
International Hazard
    
Posts: 3721
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
Wikipedia solubility pages P https://en.wikipedia.org/wiki/Solubility_table#P
or offline
Attachment: AlphabeticalSolubilityOfSalts.ods (56kB)
This file has been downloaded 437 times
EDIT: aga beat ne to it and I missed it.
[Edited on 15-3-2018 by Sulaiman]
CAUTION : Hobby Chemist, not Professional or even Amateur
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Thanks for your support guys
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
You're welcome !
You are the first of many recent newcomers who asks questions so you can actually Make Use of the answers.
P.S.
Copying your sodium thiosulphate video, it turns out that boiling S in NaOH makes a terrible smelly yellow/orange mess of polysulphides,
Na2S and Na2S2O3.
It has taken quite some effort to arrive at any quantity of white crystals of any kind.
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Yes you are correct . If you want to make sodium thiosulphate boil sulfur with sodium sulphite solution
. Boiling sulphur with sodium hydroxide will give you sodium thiosulphate but with sodium sulphide and sodium polysulphide (impossible to separate).
Even this solution is soapy to touch because sodium hydroxide does not get completely consumed in this reaction .
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sounds like a Challenge ... 
|
|
|
Akhil jain
Hazard to Self
 
Posts: 83
Registered: 19-2-2018
Member Is Offline
Mood: No Mood
|
|
Hahahkeep up the challenge
[Edited on 15-3-2018 by Akhil jain]
|
|
|
j_sum1
Administrator
       
Posts: 6333
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Quote: Originally posted by aga  |
You are the first of many recent newcomers who asks questions so you can actually Make Use of the answers. |
I will check out your dichromate ideas at some stage since that is a project I wish to do some time. I will probably drive for (NH4)2Cr2O7 or Na2Cr2O7
since I have some K salt already, the ammonium salt is fun and the Na salt has great solubility which can be useful.
Fusing with nitrate is one option but I will start with Cr2O3. If I use stainless I think I'll go full otc and oxidise with bleach as Tdep did on his
channel.
|
|
|
Fantasma4500
International Hazard
    
Posts: 1681
Registered: 12-12-2012
Location: Dysrope (aka europe)
Member Is Offline
Mood: dangerously practical
|
|
this thread seems to be the latest on chromate/dichromate so im gonna post what i got here
im analysing a solution of H2SO4 and H2CrO4, by gravity it appeared to be about 16%
but by precipitating out the chromate it appears to be much higher
the method for isolating the chromate is as follows:
react the solution with Ca(OH)2, in excess
cool the solution down in fridge, solubility of CaCrO4 is greater at lower temperature
vacuum filter it and wash it continously with cold water until the liquid coming through is no longer colored
then you react this solution with BaCl2, immediatedly BaCrO4 will precipitate out as a yellow solid
BaCl2 is added until no more precipitation occurs
dont bother trying to filter this, rather use a large volume of water and then decant it, preferably 5x the volume of water as the volume you get
after it has settled for an hour, repeat this washing 3-5 times
then simply transfer to a stainless pan and put on hotplate, keep it at low temperature so it doesnt splatter the toxic chromate around
heres some data to reference for the procedure
Ca(OH)2 + H2SO4 = CaSO4 - 0g/100mL 0*C
Ca(OH)2 + H2CrO4 = CaCrO4 - 4.5g/100mL 0*C
100g H2CrO4 + 63g Ca(OH)2 = 132g CaCrO4 + 30g H2O
100g CaCrO4 + 133g BaCl2 = 162g BaCrO4 + 71g CaCl2
100g H2CrO4 yields 214g BaCrO4
2.14g BaCrO4 = 1g H2CrO4
10mL 16% H2CrO4 = 1.6g H2CrO4 = 3.424g BaCrO4
by my experiment i ended up using 20mL of solution, which with 16% H2CrO4 yields 3.2g H2CrO4 = 6.848g BaCrO4
however, i landed on 12.5g BaCrO4- which would explain 20mL of solution is infact 29% H2CrO4- which was produced using 24.5g CrO3 per 100mL
another doable method for bulk would be to neutralize it all with NaOH, cool it down and decant the sodium chromate, while sodium sulfate mostly is
left behind as a solid
i have also used copper chromate as intermediate in seperating the chromate, its a mess to deal with and its kind of like a gel or sludge, takes long
time to filter, i would then react this sludge with NaOH in excess to give me NaCrO4 and CuO
something similar may be doable with BaCrO4
NaOH + BaCrO4 = Ba(OH)2 + NaCrO4
then gas the solution with CO2
Ba(OH)2 + CO2 = BaCO3 (ppt)
|
|
|