FlaskBreaker
Harmless

Posts: 14
Registered: 31-12-2017
Location: The 5th Dimension
Member Is Offline
Mood: Convoluted
|
|
Most Efficient Way of Making HCl?
I was wondering what the most efficient way of making hydrochloric acid is. I am already aware of the sodium hydrogen sulfate + sodium chloride
method, however, I was wondering just how 'good' of a method this is. Is there anything that is more efficient and uses fairly domestic chemicals?
I know the best way is to just buy it, however I am curious about the science of this.
Thanks,
FlaskBreaker (ScienceWithJames)
|
|
|
FrogPasta
Harmless

Posts: 1
Registered: 1-1-2018
Member Is Offline
Mood: No Mood
|
|
As far as I know the sodium hydrogen sulfate + sodium chloride is the cleanest, safest method using commonly available chemicals. There are quite a
few different ways to go about producing rather pure HCL but many of them are either dangerous or cost-ineffective. Not too long after I first got
into hobby chemistry I had this idea of utilizing the Hydrogen and Chlorine produced from electrolysis of Sodium Chloride to make HCL. As I was
reading about it the extreme danger of said reaction became obvious and I decided that purchasing smaller quantities from a local science supply was a
much better decision. Most hardware store muriatic acid in my area have additives and max out at 20% concentration.
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1640
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
hardly dangerous providing you take your time to make a dedicated system for it.
|
|
|
Iodobenzene
Harmless

Posts: 24
Registered: 27-12-2017
Location: In solution
Member Is Offline
Mood: Strongly Acidic
|
|
You should try salt and sulfuric acid:
2NaCl + H2SO4 ---> 2HCl + Na2SO4
You put in a round bottom flask with two necks a NaCl solution.
You put an addition funnel in the first neck with H2SO4.
in the Second neck you put a stopper with tube and you put the end of it in a Becker containing distilled water.
You add the H2SO4 dropwise using the funnel.
in the Becker you'll get an HCl solution free from impurities.
[Edited on 1-1-2018 by Iodobenzene]
[Edited on 1-1-2018 by Iodobenzene]
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The industrial route is to split brine into H2, Cl2 and NaOH by electrolysis and to combine the H2 and Cl2 in a combustion tube. This is considered
impractical for the home chemist, but I've seen writeups involving much more complicated and dangerous pieces of apparatus... how practical it is
depends on how badly you want to make some HCl.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Here's an illustration of Iodobenzene's setup:
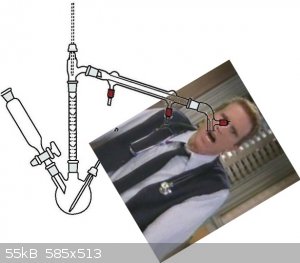
Anyone? I hope I'm not the only one that remembers that show.
|
|
|
happyfooddance
National Hazard
   
Posts: 530
Registered: 9-11-2017
Location: Los Angeles, Ca.
Member Is Offline
Mood: No Mood
|
|
Our stomachs use HCl to break down food. Vomit is a viable route.
|
|
|
Brom
Hazard to Self
 
Posts: 94
Registered: 19-7-2015
Member Is Offline
Mood: No Mood
|
|
Becker ha ha. I never watched the show but I remember when it was on
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Hahaha. Is that a glass rod for stirring and breaking up lumps?
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Not sure, I just pulled that off Google. It might be a "sparge tube" (probably not the right term), for bubbling gas into the flask to help stir or as
part of the reaction.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
A glass rod through a septum would be ideal in that sort of apparatus....
|
|
|
Iodobenzene
Harmless

Posts: 24
Registered: 27-12-2017
Location: In solution
Member Is Offline
Mood: Strongly Acidic
|
|
ahahha i'm sorry for my bad English.
i meant a cork stopper with a hole,and in that hole you put a tube to gurgle the gaseous HCl in a becher filles with water.
[Edited on 2-1-2018 by Iodobenzene]
|
|
|
unionised
International Hazard
    
Posts: 5128
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Iodobenzene  | ahahha i'm sorry for my bad English.
i meant a cork stopper with a hole,and in that hole you put a tube to gurgle the gaseous HCl in a becher filles with water.
[Edited on 2-1-2018 by Iodobenzene] |
Your English isn't a problem.
(But, just to let you know, the word is "beaker")
Dissolving very soluble gases like HCl is tricky.
You need an anti "suck back" device like the one(s) here
http://www.sciencemadness.org/talk/viewthread.php?tid=30479&...
|
|
|
Iodobenzene
Harmless

Posts: 24
Registered: 27-12-2017
Location: In solution
Member Is Offline
Mood: Strongly Acidic
|
|
Thanks,i'll check it out.
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Hi swiming pool regulator ( SO4HNa) + sodium chloride, is more OTC ,in my country sulfuric acid is forbbiden. And apparatus appropiate sure.
|
|
|
FlaskBreaker
Harmless

Posts: 14
Registered: 31-12-2017
Location: The 5th Dimension
Member Is Offline
Mood: Convoluted
|
|
Quote: Originally posted by GrayGhost-  | | Hi swiming pool regulator ( SO4HNa) + sodium chloride, is more OTC ,in my country sulfuric acid is forbbiden. And apparatus appropiate sure.
|
That's too bad, considering how useful of a reagent H2SO4 is.
I am having trouble making HCl the NaHSO4 + NaCl route. I used 1 gram of NaHSO4 and 2.3 grams of NaCl (This is a 1:1 ratio). I used a very small
amount of water to allow the chemicals to interact. I started heating and saw a vapor beginning to form. (I am suspicious of this being steam) Some
bubbles formed at the inverted funnel trap. I monitored the PH, however it seemed to stay the same. I may try this again, however at this time, it's
way to cold outside to do this again. (I do not have a fume hood yet.) I suspect this is because it was too cold outside to maintain the temperature
needed to drive the reaction.
|
|
|
Iodobenzene
Harmless

Posts: 24
Registered: 27-12-2017
Location: In solution
Member Is Offline
Mood: Strongly Acidic
|
|
Quote: Originally posted by GrayGhost-  | | Hi swiming pool regulator ( SO4HNa) + sodium chloride, is more OTC ,in my country sulfuric acid is forbbiden. And apparatus appropiate sure.
|
Then try to heat a 38% HCl solution (which is sold at hardware stores) and gurgle the vapours in distilled water.
Anyway,what's your country?
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Iodobenzene  | Quote: Originally posted by GrayGhost-  | | Hi swiming pool regulator ( SO4HNa) + sodium chloride, is more OTC ,in my country sulfuric acid is forbbiden. And apparatus appropiate sure.
|
Then try to heat a 38% HCl solution (which is sold at hardware stores) and gurgle the vapours in distilled water.
Anyway,what's your country?
|
Hi, I live in ass of world ( Argentina) , muriatic acid was sold in hardware store to 2012 , so easy like buy screws or nuts. Today is banned to
common people. You need permission.
Here do not exist meth, but cooking cocaine, base pasty of cocaine is reacted with chloridric acid/ muriatic.
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
That is reason for government forbbiden that chemical, I am amateur chemist
is my sincered aclaration.
I buy diluited muriatic acid mixtured with fosforic acid . Is a waste .
[Edited on 3-1-2018 by GrayGhost-]
|
|
|
Iodobenzene
Harmless

Posts: 24
Registered: 27-12-2017
Location: In solution
Member Is Offline
Mood: Strongly Acidic
|
|
you can do the heat up method also with the HCl H3PO4 mixture,try it.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
I find this to be one of the coolest path, but not orthodox that may result in an unexpected strong acid mix. It starts by following my previous
described path to HOCl based on CaCl2 + NaOCl (aq) + CO2, see http://www.sciencemadness.org/talk/viewthread.php?tid=71477#... ([Edit] or better, per comments on divalent (or higher valences) chlorides below
contributing to the 'activity coefficient' (see http://www.csun.edu/~hcchm003/321/321100313.pdf) of the mix, convert the NaOCl to Mg(OCl)2 by adding MgSO4 to 2 NaOCl and freeze out the
Na2SO4.10H2O, the action also removing water):
CaCl2 + 2 NaOCl (aq) = Ca(OCl)2 + 2 NaCl
H2CO3 + Ca(OCl)2 = CaCO3(s) + 2 HOCl
Convert the HOCl/NaCl mix to HCl/NaCl and some even more powerful HClO3 by placing in sunlight (an optional catalyst is a very small amount of lemon
juice as a citric acid source, and/or vinegar (for acetic acid) or use NaNO2 or NaNO3 or even added N2O gas, but the latter requires UV light, which
is said to be 80% reflected off of snow, along with a bit of HClO4 formation per reference below). The added NaCl impurity interestingly increases the
activity coefficient and, as I also previously noted, the HCl/NaCl mix can significantly (in higher NaCl concentrations, or better, divalent chlorides
like MgCl2) act as a much stronger acid (a citation of a source claiming that 2M HCl in 3M MgCl2 or CaCl2,..behaves like 7M HCl at http://www.sciencemadness.org/talk/viewthread.php?tid=38378#... ).
An extract from a prior thread on this topic, with many other cited conventional paths (see http://www.sciencemadness.org/talk/viewthread.php?tid=17638 ):
Quote: Originally posted by AJKOER  | Actually, this suggested preparation is based on actual (and unexpected) observed behavior of such a oxidizing solution I once created via photolysis
(it bleached the hell out of part of the flooring in an old shower in my basement that I have been unable to replicate since with strong chlorine
bleach). I did, however, prepare the Hypochlorous acid differently, via acetic acid on NaClO. The Sodium acetate was not removed as there was no
distillation performed.
It should be noted that with sunlight, the reaction:
HOCl --uv--> HCl + O
has been reported to proceed more rapidly in the presence of Tartaric or Citric acid, which apparently act as catalysts (see "A comprehensive treatise
on inorganic and theoretical chemistry", Volume 2, by Joseph William Mellor, top of page 82, where to quote: "According to C. Lowig,{27} bromine water
in light behaves in a similar way to that of chlorine water, but as J. M. Eder showed, bromine water is much less sensitive to light in that it
decomposes with but one-sixth or one-twelfth the speed of chlorine water. The presence of tartaric or citric acid accelerates the decomposition of
chlorine or bromine water in light." Link: http://books.google.com/books?pg=PA82&lpg=PA82&id=An... ).
My recent readings also note in general that organic compounds in water generally (and acetic acid, I would guess in particular) appear to promote the
formation of hydroxyl radicals (and therein, HClO3 in addition per the action of radicals discussed above). See, for example, "Chemistry of Marine
Water and Sediments", edited by Antonio Gianguzza, Ezio Pelizzetti, Silvio Sammartano,.. , page 90, at https://books.google.com/books?id=DacPIDoNaNQC&pg=PA90&a... detailing the formation of H2O2, which can produce hydroxyl radical under
photolysis.
Now, I also even recall one paper discussing the formation of even perchlorates via photolysis. See, for example, "Perchlorate production by
photodecomposition of aqueous chlorine solutions", abstract at http://www.ncbi.nlm.nih.gov/pubmed/22962844 .
So bottom line in my opinion, there appears to be some observations and theory suggesting that one may be able indeed to create quite a strong acid
via HOCl, an organic catalyst (I would revise my suggested preparation above with the addition of a mix of critic or acetic acid on NaClO), good uv
exposure (using the presence of snow , for example) and a good amount of time.
[Edited on 20-1-2015 by AJKOER] |
[Edited on 4-1-2018 by AJKOER]
|
|
|
GrayGhost-
Hazard to Self
 
Posts: 61
Registered: 31-10-2017
Location: Argentina
Member Is Offline
Mood: No Mood
|
|
Well is not scientific source but apparently work.
https://idl-reporteros.pe/wp-content/uploads/2012/02/metodo-...
https://idl-reporteros.pe/wp-content/uploads/2012/02/metodo-...

|
|
|