Mr.Pink
Harmless

Posts: 3
Registered: 4-11-2017
Member Is Offline
Mood: No Mood
|
|
Help with schoolwork
So in class we did a titration experiment. Im doing the calculations for part two and the teacher asked for molarity of acetic acid. But we didnt use
a specific volume of vinegar(well we did but we diluted it to an unknown volume). We massed the vinegar for the calculations. But im at a loss as how
to go from the concentration of acetic acid to its molarity...iv run through a couple different calculations but none worked out.
We put 0.1 mL of vinegar in a flak and added 1 drop of phenolphthalein and rinsed down the sides of the glass with deionized water. Then titrated it
with a 0.1M solution of NaOH.
Ill attach pics of the data because its a bit much to type out
 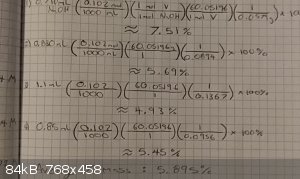
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
The definition of molarity is moles of substance per liter of solution. Can you explain why you placed each term in the first line of your
calculations?
|
|
|
Mr.Pink
Harmless

Posts: 3
Registered: 4-11-2017
Member Is Offline
Mood: No Mood
|
|
I dont really understand you question...but the oic on the left is values used in the experiment and on the right iv calculated the mass percent of
the vinegar. Im struggling on finding the molarity of the vinegar. The 0.1 mL is inaccurate due to the water rinse. So i figured i would have to use
it mass percent which the average states in every liter of vinegar is approximately 59 mL of acetic acid and work to find its molarity from there.
...im guessing that maybe transfer the mass percent into grams by useing its density in the calculation. Then to moles then to M. Yes?
|
|
|
Mr.Pink
Harmless

Posts: 3
Registered: 4-11-2017
Member Is Offline
Mood: No Mood
|
|
I just figured it out...i was way overthinking as usual. I take the mass of the weighed vinegar and using the mass percent determine the actual mass
of the acetic acid. Use that to determine moles per 0.1mL then multiply that by 10,000 to get the molarity.
|
|
|