pmanavado
Harmless

Posts: 1
Registered: 18-10-2017
Member Is Offline
Mood: No Mood
|
|
2-(benzhydrylthio)acetic acid synthesis
Hello:
Does anyone know how i could go about making 2-(benzhydrylthio)acetic acid. I have searched the internet and was not able to find anything about it's
synthesis.
I do appreciate all help
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'm not really sure what compound that is... what is the IUPAC name?
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 351
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by pmanavado  | Hello:
Does anyone know how i could go about making 2-(benzhydrylthio)acetic acid. I have searched the internet and was not able to find anything about it's
synthesis.
I do appreciate all help |
This substance is a precursor to Modafinil and Andrafinil. There's a reference to it's synthesis at Rhodium pages, I am attaching below.
If you intend to synthesize Modafinil or Andrafinil there's easiest ways to do that without the need to make 2-benzhydrylthioacetic acid.
Do a search here at Sciencemadness and you will find a lot of references I'd posted some time ago in another thread.
There's another easy way to synthesize it starting from the Bunte Salt too, at prepublication section, written by KMnO4.
Attachment: modafinil V.pdf (204kB)
This file has been downloaded 671 times
|
|
|
j_sum1
Administrator
       
Posts: 6377
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
It looks like this:
https://www.scbt.com/scbt/product/2--diphenylmethyl-thioacet...
And I have no idea where to start.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
Hm... diphenylmethyliodide would form a thioether with 2-thioethanol, which could then be oxidized with chromic acid. Benzophenone would probably be a
good starting point.
|
|
|
Metacelsus
International Hazard
    
Posts: 2544
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
The chromic acid would likely oxidize the thioether to at least the sulfoxide (which might be OK given the intended final product) and possibly the
sulfone (which would be unwanted). Source
I would recommend the Bunte salt method, which is described on this forum.
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
I'm pretty sure the thioether would oxidize slowly, especially in the cold, but the alcohol/aldehyde would react almost instantly in cold acetone.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
http://www.sciencemadness.org/talk/viewthread.php?tid=66274#...
Quote: Originally posted by Chemi Pharma  |
If you intend to synthesize Modafinil or Andrafinil there's easiest ways to do that without the need to make 2-benzhydrylthioacetic acid.
|
really ?
|
|
|
Chemi Pharma
Hazard to Others
  
Posts: 351
Registered: 5-5-2016
Location: Latin America
Member Is Offline
Mood: Quarantined
|
|
Quote: Originally posted by Chemi Pharma  |
If you intend to synthesize Modafinil or Andrafinil there's easiest ways to do that without the need to make 2-benzhydrylthioacetic
acid.[/rquote]
really ? |
What I mean @Cureus is the fact you can do Modafinil and Andrafinil synthesis without producing benzhydrylthioacetic acid as an intermediate, such
Rhodium's recipe done.
You can sinthesize the amide directly, starting from the same bromo-diphenyl-methane, like this:
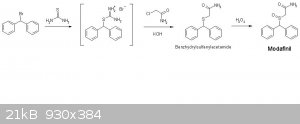
Also, Via Bunte salt the intermediate formed is the amide and not the acid.
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2836
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
So have we decided that modafinil is not recreational? Cool.
Thioglycolic acid is used in hair removers:
http://en.wikipedia.org/wiki/Thioglycolic_acid
I believe that it can be converted to its amide by heating the ammonium salt, similar to other amide preparations. From thioglycolamide all you need
to do is react with benzyhydryl halides and oxidize the resulting thioether.
As for the extraction of thioglycolate from hair removers -- perhaps you can precipitate the calcium salt "CaSAcO" and then liberate the thioglycolic
acid with H2SO4.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by clearly_not_atara  | | As for the extraction of thioglycolate from hair removers -- perhaps you can precipitate the calcium salt "CaSAcO" and then liberate the thioglycolic
acid with H2SO4. |
ammonium thioglycolate is directly available as perm salt -https://en.wikipedia.org/wiki/Ammonium_thioglycolate
|
|
|