Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
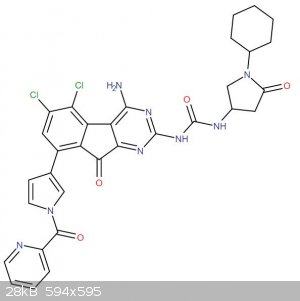
Synthesis of a biologically active compound
As you can see in the attached image, the molecule is rather big.
I have been modelling molecules to bind TRAAK channels, either as agonists or antagonists. The following molecule was one of the best ones. Is it even
feasible for home chemists? Evidently, with the suitable reagents and SMs, it's not rocket science to put this molecule together (apart from that
inner fluorene-like thing), because it's just composed of lots of small bits - urea, amides, etc. all easy to make (as long as you have the
cyclohexane, the suitable diaminoacids, etc.).
Therefore, my question is not "how u mayk dis" because, apart from the central bit where I'm actually curious (no hits on reaxys or elsewhere), it is
trivial.
[/digression]
In any case, this is a chance for a more open question: what are suitable readily available starting materials (for home chemists) for this reaction?
Pyrimidines, pyrrole (either with a halogen or some way to join to the other aromatic ring)...?
PS. Yes, I know that in silico modelling does not immediately prove a very high bioactivity and yes, I know that even if it did, this drug would have
quite a low bioavailability. But I think that as a synthesis it's quite interesting and that its potential use is already a good enough incentive.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
Texium
Administrator
       
Posts: 4619
Registered: 11-1-2014
Location: Salt Lake City
Member Is Offline
Mood: PhD candidate!
|
|
Wow, that looks like a bear. Not sure if it's of any help, but the first thing that popped into my mind when I thought about synthesis of custom
fluorene-like compounds was the Borsche–Drechsel Cyclization which forms carbazole from phenylhydrazine and cyclohexanone. Still you'd then have the
problem of somehow replacing that amine with a carbonyl, but perhaps if you could start with an appropriately substituted phenylhydrazine and
cyclohexanone you could at least get to that basic structure.
|
|
|
Dr.Bob
International Hazard
    
Posts: 2753
Registered: 26-1-2011
Location: USA - NC
Member Is Offline
Mood: Mildly disgruntled scientist
|
|
Looks like a graduate research target.
I can see two ways to get to the left (south west) part, either couple the pyyrole, then link the picolinoyl acid chloride (which is one of the worst,
least soluble acid chlorides ever), or better yet, try to make that piconoyl amide of 3-halo pyrrole, and then convert that to the boronic acid or
similar coupling agent.
Similarly, the top right (north east) part might be best made separately as the N-cyclohexyl 3-aminopyrrolidine, then make the isocyanate and couple
that to the core amine, which would require that the other amine be protected or maybe hidden there as a nitro group. You might be able to use the
the amines, and some other reagent, like the nitrocarbonate or even Carbonyl diimidazole to make the urea, but they are tougher on that type of
heteroamines, I believe.
The core looks challenging, but doable with enough work. But all together it looks like about 15 steps or more to the final product. That would
cost a lot of time, chemicals and money. Maybe better to put into a virtual pool in some academic center's database and try to get them to pick it
as a hit. Not sure if there is a good place to post structures to get people to adopt them...
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Now, this is the kinda project, that big pharma can do........ like no-one else.
In fact, they may have, made this stuff, already!
But, how to find out?
Might I suggest, you put on your magnifier glasses, and camp out at a real library. Like at U.C. Berkeley maybe? Usta be, once you got inside, you
could stay all night. Stay for days, in fact.
The big boys, would put a bunch of people on something like this (just doing the research) and have them pound on it, until they had some answers.
To me, it looks like it might be an opioid. Some areas are remind me of U47700. The legality of such materials, is rapidly retreating. https://en.wikipedia.org/wiki/U-47700
[Edited on 19-7-2017 by zed]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
It's not an opioid or, if it was, it would be entirely unexpected.
I based this molecule (very loosely) around lamotrigine and sipatrigine, checking whether some kind of modification to the central section could
enhance the effects (and the new ring did). That was the initial idea for the pharmacophore when I optimised the structure.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
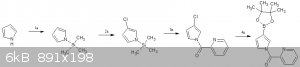
from left to right
a) synthesis of the pyrrole moiety:-
1.silylation of pyrrole -http://www.sciencedirect.com/science/article/pii/S0040403905...
2.chlorination - | Quote: | | halogenation generally occurs at the 2-position, but can also occur at the 3-position by silation of the nitrogen |
https://en.wikipedia.org/wiki/Pyrrole#Reaction_of_pyrrole_wi...
3.acylation -http://sciforum.net/conference/ecsoc-15/paper/632
4.borylation - http://www.organic-chemistry.org/abstracts/lit2/187.shtm
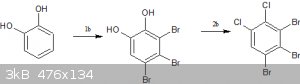
b) synthesis of the halogenated catechol:-
1.tribromination of catechol-http://pubs.acs.org/doi/abs/10.1021/ja01145a519
2.appel reaction on catechol-https://en.wikipedia.org/wiki/Appel_reaction
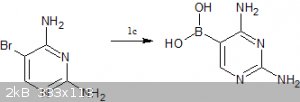
c)synthesis of pyrimidine:-
1.borylation of 5-bromopyrimidine-2,4-diamine -http://www.organic-chemistry.org/abstracts/lit5/336.shtm (the 5-bromopyrimidine-2,4-diamine has to be bought since I don't know how to make it
 ) )
d)synthesis of right side
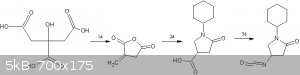
1.citric acid to itaconic anhydride -http://orgsyn.org/demo.aspx?prep=CV2P0368
2.reaction of anhydride with cyclohexylamine - | Quote: | | Place anhydride and cyclohexylamine (9.15 g, 92.2 mmol) and heat to 16O'C for hours. Cool to give 18.8 g (96%) of l-cyclohexyl-5-
oxo-pyrrolidine-3-carboxylic acid |
http://www.freepatentsonline.com/WO2006049952A1.html (they use itaconic acid but anhydride should work even better  ) )
(1-cyclohexyl-5-oxo-pyrrolidine-3-carboxylic acid is availabe commercially,so you could directly start from here  ) )
3.schimdt reaction to make the isocyanate - | Quote: | | Schmidt reaction, a reaction where a carboxylic acid is treated with ammonia and hydrazoic acid yielding an isocyanate. |
https://en.wikipedia.org/wiki/Isocyanate#Rearrangement_react...
e)final convergent synthesis
1. suzuki coupling of borylated pyrrole with 3,4,5-tribromo-1,2-dichlorobenzene(5th Br gets coupled since its the most easily accessible due to steric
hindrance)
2.synthesis of the flourenone -http://www.organic-chemistry.org/abstracts/lit4/922.shtm
3.formation of the urea linkage (the NH2 on the right of the pyrimidine reacts with the isocyanate since its more easily accessible due to
steric hindrance)
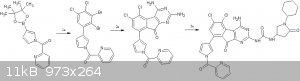
[Edited on 30-7-2017 by CuReUS]
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
Congratulations, this is very impressive! Just wondering, do the Cl and Br in the last Suzuki coupling really cause significant enough steric
hindrance? I would have assumed that you would get some regioselectivity but not that much at all.
As for e)2., remember that it i not a fluorenone but rather has a pyrimidine ring on one side. The NH2- could also change the outcome/reaction? This
is one of the cases where actually trying to do it seems the only way to see what happens.
Overall, impressive.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Eddygp  | | Congratulations, this is very impressive! Just wondering, do the Cl and Br in the last Suzuki coupling really cause significant enough steric
hindrance? I would have assumed that you would get some regioselectivity but not that much at all. |
thank you ! 
do you mean the pyrrole coupling with tribromo dichlorobenzene ? Because if you noticed carefully, I purposely put a pinacol boron on the pyrrole
rather than just B(OH)2 to make it extra bulky so that there was steric hindrance from this side as well  
|
|
|
|