Doctor Cat
Harmless

Posts: 25
Registered: 9-8-2015
Member Is Offline
Mood: No Mood
|
|
Help with Target Molecule
I want to synthesise the following target molecule but I am not sure if the reactions are possible. I decided to have a second opinion.
I still have to work on the conditions of the reaction but that is not something that literature can not provide.
Thanks in advance.
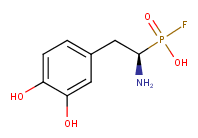
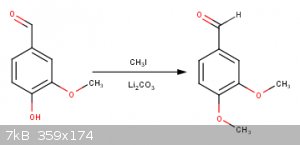
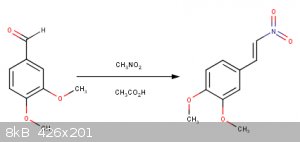
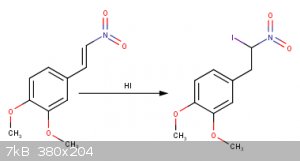
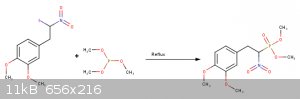

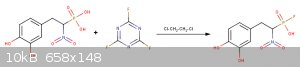
Im not sure if the catechol ring will also get fluorinated...
Some Refereneces:
A NEW MICROSCALE METHOD FOR THE CONVERSION OF PHOSPHORUS OXYACIDS TO THEIR FLUORINATED ANALOGUES, USING CYANURIC FLUORIDE IN SOLUTION AND ON SOLID
SUPPORT. Rikard Warme and Lars Juhlin. Phosphorus, Sulfur, and Silicon, 185:2402–2408, 2010
https://en.wikipedia.org/wiki/Michaelis%E2%80%93Arbuzov_reac...
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
When you add HI across the double bond, are you sure the iodide will go on that carbon? I thought the hydrogen would go on that carbon to give a
resonance-stabilized benzyl cation. But I'm not an organic chemist.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Doctor Cat
Harmless

Posts: 25
Registered: 9-8-2015
Member Is Offline
Mood: No Mood
|
|
Yes that is correct according to Markovnikov's rule but in the products there should be the two of them but in different proportions... I was also
thinking of attempting an anti markovnikov reaction with HBr on hydrogen peroxide... I'm not sure if this can be performed with HI instead of HBr but
I don't think so.
|
|
|
DraconicAcid
International Hazard
    
Posts: 4356
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
That has nothing to do with Markovnikov's rule, but the stability of the intermediate.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
Doctor Cat
Harmless

Posts: 25
Registered: 9-8-2015
Member Is Offline
Mood: No Mood
|
|
I think it does due to more stability of the carbonium ion formed but please correct me if I am mistaken
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I'm pretty sure the selectivity in that third reaction will exceed 90% for putting the iodine on the benzylic carbon. A carbocation adjacent to the
nitro group is not stable.
Consider a Baylis-Hilman-like reaction with benzyl fluorochlorophosphonate on methylenedioxynitrostyrene, perhaps? I don't know how stable the
fluorophosphonate group is at all.
|
|
|
Doctor Cat
Harmless

Posts: 25
Registered: 9-8-2015
Member Is Offline
Mood: No Mood
|
|
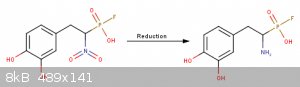
It took me some time but I finally found out a described procedure for making (2-bromo-2-nitroethyl)benzene ( http://etd.library.vanderbilt.edu/available/etd-03302010-153... ) (pp.154) but they did it at -78°C which is not a possibility at all for me.
@clearly_not_atara I am not familirized with the Baylis-Hilman reaction, can you give me an insight on your thoughts?
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Doctor Cat, this is a very strange compound you want to make. Would you please tell us of your motivations?
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Doctor Cat
Harmless

Posts: 25
Registered: 9-8-2015
Member Is Offline
Mood: No Mood
|
|
Yes of course. I am looking for more potent inhibitors of the enzyme Aromatic L-amino acid decarboxylase related with the treatment of parkinson
disease. Current inhibitors are not very potent or do not cross the blood-brain barrier.
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Keep us posted! My friend with Parkinsonism may be helped by this...
"When you let the dumbasses vote you end up with populism followed by autocracy and getting back is a bitch." Plato (sort of)
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2799
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
| Quote: | | they did it at -78°C which is not a possibility at all for me. |
This is a common myth. -78ºC is popular exactly because it is the lowest temperature easily obtained in a laboratory. It is achieved by mixing a
large amount of dry ice into chilled water-free acetone. That's at least as OTC as anything else we do here, you just need insulated vessels to
contain it.
| Quote: | | @clearly_not_atara I am not familirized with the Baylis-Hilman reaction, can you give me an insight on your thoughts? |
The Baylis-Hilman reaction allows the SN1 addition of certain electrophiles to an electron-deficient alkene geminal to the EWG. Usually the
electrophile is a carbonyl group or imine, but in this case I was hoping a phosphonic halide would work. This would transform 1-nitro-2-phenylethylene
into 1-nitro-1-benzyloxyfluorophosphonoyl-2-phenylethylene.
This is an "umpolung" reaction which is made possible by using a nucleophile which reversibly forms an unstable intermediate by reacting with the
alkene in a Michael-type addition. Usually the nucleophile is DABCO. The balancing act is then to choose an electrophile which is strong enough to
react with the DABCO-alkene complex, but which won't react with DABCO itself. So organic halides are usually out, because of quaternization, and weak
electrophiles like esters won't react at all. Phosphonic chlorides may or may not react with DABCO but I'm pretty sure they'll attack the transient
carbanion.
Note that I'm assuming some basic familiarity with chemistry because phosphonic acids, especially their acyl fluorides, can be absurdly dangerous and
cause a horrifically painful death worthy of a Greek myth (also worthy of the international conventions which prohibit their use). It is not advisable to
work with such chemicals if you don't know what you're doing.
[Edited on 7-2-2016 by clearly_not_atara]
[Edited on 7-2-2016 by clearly_not_atara]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Umm. Back in the day, I had a friend with Parkinson's symptoms.
Loading up on precursors for the body's natural pathway to Dopamine, seemed to help her a lot. Tyrosine, orally, several times per day, seemed to
relieve a lot of her symptoms.
Also of Note: The tripeptide PLG was getting rave reviews in the literature at that time. Might require insufflation or parenteral administration.
Pharm, wasn't very interested in the treatment......probably not patentable, and short shelflife. They were looking for alternatives in the same
ballpark, that were a little sexier.
I'll go take a relook at PLG. It was reputed to have dramatic anti-Parkinson's effects.
Yup! Still some information out there. With today's technology, probably an easy make. https://en.wikipedia.org/wiki/Melanocyte-inhibiting_factor
[Edited on 7-2-2016 by zed]
[Edited on 7-2-2016 by zed]
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
Please forgive me if I've misunderstood something in your posts, but inhibition of aromatic L-amino acid decarboxylase (AAAD) in the central nervous
system is not a mechanism for the treatment of Parkinson's disease (PD). The role of AAAD is to produce dopamine by
decarboxylation of its immediate precursor, L-DOPA (3,4-dihydroxyphenylalanine). If you inhibit this enzyme, there will be no production of dopamine
in the CNS - and since the symptoms of PD arise primarily because of a lack of dopamine in certain regions of the brain, inhibiting its production can
ONLY make things worse. Many therapies for PD are heavily targeted at increasing the supply of dopamine in the CNS - hence the therapeutic
use of L-DOPA (or the administration of tyrosine, which is the biological precursor to L-DOPA).
Now, peripheral AAAD inhibitors, such as carbidopa, are critical drugs for the treatment of PD - but they are important precisely
because they do not penetrate the blood-brain barrier. When you take L-DOPA, it can be metabolised easily outside the brain, which has two
effects - one, less reaches the brain, where it is needed; and two, there are extensive peripheral side effects. By taking an AAAD inhibitor which
cannot enter the brain, you can prevent peripheral metabolism of L-DOPA, thus reducing these problems.
So, in short, peripheral inhibition of AAAD is critical to achieving effective treatment of PD with L-DOPA; central inhibition of
AAAD would be entirely counterproductive.
---
As an aside, it's rarely a good idea to experiment on humans with poorly studied compounds. Whilst it is entirely true that some compounds, which have
never been tested in humans, will go on to become useful and effective drugs, you have to remember - of the millions of compounds known to us
(CAS indicates they catalogue over 106 million chemical substances, and add some 15,000 each day; a number of sites suggest that the number of
well-defined substances is more like 10 million), only a very small number are clinically useful - and a heck of a lot are actively harmful. It's
entirely natural - but completely unreasonable - to approach the treatment of a medical condition with the attitude of "it might help" - because that
fails to account for the enormous probability that it won't help, as well as the huge probability that it will actively harm. Don't
get me wrong, I fully understand desperation when faced with debilitating or terminal disease, I've been through it personally (both as a patient with
chronic pain and a relative of cancer patients), but it's important to remember that - no matter how good your intentions, and no matter how good the
preliminary studies you're relying on are - you're massively more likely to harm than to help when attempting to apply untested "therapies"
to the treatment of a disease.
You have to remember that the tiniest change to an effective drug can turn it into an inert lump, have it target a different system, or cause it to
become a dangerous toxin. Enantiomers can have absurdly different properties; different salts of the same drug can have different properties; and even
different crystalline polymorphs of the same compound can have different pharmacological properties.
It's very easy to look through the literature and find all sorts of "highly promising" compounds, which have this or that effect on some disease or
other. But the difference between "causes cellular response in a dish" or "effective in an animal model" and "effective (and safe) drug" is enormous.
Compounds which show great promise in one stage of testing can fail spectacularly (and, just to emphasise the point, this very recent tragedy in a phase 1 trial was, according to Nature, testing a drug with
potential utility in the treatment of PD!) as you move through the various stages of testing required to get a drug to market - for reasons of poor
effectiveness, intolerable or dangerous side effects, or even that it simply doesn't move through the body (absorption, distribution, metabolism or
excretion) like it needs to in order to be useful. Certainly profitability is an issue - but it usually influences which diseases a company decides to
focus on (and developing an effective treatment for age-related diseases like PD is very profitable) rather than which specific drugs they
choose to pursue (because it's easy enough to tweak a molecule just a little in order to make it patentable - and probably improve on the properties
of the unpatentable one as an added bonus). Then, of course, there's also the enormous body of basic research which is just flat-out wrong - there are so many reasons that the literature can be misleading or outright wrong (most of which are entirely reasonable outcomes
from the way the scientific process works, and have nothing to do with deliberate fraud or deception), that any attempt to extrapolate from a small
body of positive studies (or from purely bench studies) to "this must work" or "well, it might help, so what's the harm?" is almost certainly doomed
to failure (and, probably, to causing more harm than good).
Long story short - seek the advice of appropriately qualified physicians (ideally specialists in the appropriate area - a neurologist who specialises
in movement disorders in the case of PD), and if you (or your friends or relatives, as appropriate) are not getting satisfactory results from the
treatments that the doctors can offer, you should seek out (with your doctor's assistance, where possible) real clinical trials for which you might be
eligible, rather than attempting to self medicate with untested or unknown "therapies". By enrolling in a trial, you know that at least some safety
testing has been done; that the drugs you're taking are produced under carefully regulated manufacturing conditions; that you'll be taking the drugs
under the close supervision of highly trained medics (often the care and attention received by clinical trial patients far exceeds that which you can
get from even the best "regular" doctors); and that the effectiveness of the drug is being properly studied and the results will, perhaps, help you -
and others like you - in the longer term.
|
|
|
j_sum1
Administrator
       
Posts: 6334
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
Thorough as always, ziq. However, I believe that the OP was investigating for the chemical challenge rather than for self-administration or any other
kind of use.
|
|
|
ziqquratu
Hazard to Others
  
Posts: 385
Registered: 15-11-2002
Member Is Offline
Mood: No Mood
|
|
You're entirely right, j_sum - I may have read more into the posts above (and focused too much on zed's comments about tyrosine, rather than Doctor
Cat's posts) than was warranted. If so, I apologise!
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
http://www.tandfonline.com/doi/abs/10.3109/00207459008994244
http://www.ncbi.nlm.nih.gov/pubmed/2502304
Wurtman wrote extensively on nutrient supplementation and increased neurotransmitter release. It isn't rocket science.
Since the side effects of L-Dopa and its ilk are fairly unpleasant, many people have resorted instead to Tyrosine supplementation, to decrease
Parkinson's symptoms. Seems to work well for some people.
MIF is also an interesting alternative. Of interest too, is its effect on opiate receptors.
I suppose, I am actually responding to Chemrox. He has a friend who might benefit from the information.
[Edited on 9-2-2016 by zed]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. Been a while. Recently tried ingesting Olive leaf extract, for an unrelated condition.
Turned out to have an unexpected quality.....Mood elevation!
It's structure suggests it may be boosting brain dopamine levels. Or faking receptors out.
Turns out a major component of Olive leaf extract, is Oleuropein. An ester of 3-4 Dihydroxy-phenethyl alcohol. Awfully close to the structure of
Dopamine itself.
https://en.wikipedia.org/wiki/Oleuropein
[Edited on 30-5-2017 by zed]
|
|
|
Chemetix
Hazard to Others
  
Posts: 376
Registered: 23-9-2016
Location: Oztrayleeyah
Member Is Offline
Mood: Wavering between lucidity and madness
|
|
It's a bit old now but I just want to say the HI addition has the problem of chewing off your 3-4, methyl ethers.
But the motivation for the OP's TM seems one of those 'let's search for something we can sell rather than investigate something we know works'. I
recall seeing many reports about a certain methylenedioxy compound having excellent results with PD, and because it's non patentable...no commercial
interest.
|
|
|
Alice
Hazard to Others
  
Posts: 111
Registered: 11-5-2015
Member Is Offline
Mood: No Mood
|
|
In order to be CNS active, the compound has to cross BBB. Therefor the compound needs to be either lipophilic or utilze a dedicated transporter. See
http://molpharm.aspetjournals.org/content/61/4/729.short.
The reason why L-DOPA crosses BBB is not lipophilicity but the transporter mechanism. So the question for the fluorophosophonic acid analogue is if
there is a suitable transporter. Are there any comparable compounds known where the transporter mechanism has been shown to work?
[Edited on 31-5-2017 by Alice]
|
|
|
zed
International Hazard
    
Posts: 2284
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Ummm. More info on Olive Oil/Oleuropein.
https://www.scirp.org/journal/PaperInformation.aspx?PaperID=...
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3892135/
Interesting papers out there, but some are from odd places. Papers from some regions of the world, have decreased credibility.
[Edited on 2-6-2017 by zed]
|
|
|
Outer
Harmless

Posts: 38
Registered: 24-11-2008
Member Is Offline
Mood: No Mood
|
|
I think that your molecule cannot exist, so as it contains both strongly electrophilic (phosphorous halogenide) and nucleophilic (hyroxy- end,
especially, amino-groups) fragments which will quickly react each with other.
|
|
|