ovit.us
Harmless

Posts: 3
Registered: 18-3-2017
Member Is Offline
Mood: No Mood
|
|
Enamine formation help
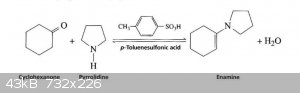
I think I'm having issues forming an Enamine. This is the procedure I've been following:
An apparatus was setup for the azeotropic removal of water using a Dean-Stark water-trap and Dimroth refux condensor. Into the 250ml round-bottom
flask, 40ml of Pyrrolidine, 35ml of Cyclohexanone, 500mg of P-Toluenesulfonic acid, and 100ml of Toluene was added. This was heated with stirring for
about 5 hours until ~6ml of water separated.
The reaction mixture was slightly yellow, but had what appeared to be a white precipitate floating in it. This has happened every time this reaction
was performed. Is this what should occur? I have done a similar reaction involving Morpholine, and it has always appeared clear after reflux (although
still yellowish).
Following this I would performed a distillation and the result would be a clear liquid, which would gradually change back to yellow. With the
Morpholine reaction, this distillation liquid would remain stable, and would not change color.
Any ideas? I'd like to know if I've missed some vital step or am going about this incorrectly. I could take a picture of the reaction, it just appears
yellow with a cloudy substance suspended in it.
|
|
|
Corrosive Joeseph
National Hazard
   
Posts: 915
Registered: 17-5-2015
Location: The Other Place
Member Is Offline
Mood: Cyclic
|
|
Attachment: Imines, enamines and oximes.pdf (337kB)
This file has been downloaded 706 times
/CJ
Being well adjusted to a sick society is no measure of one's mental health
|
|
|
ovit.us
Harmless

Posts: 3
Registered: 18-3-2017
Member Is Offline
Mood: No Mood
|
|
Thanks, I don't see my particular reaction in that document, but good info to have.
This is what my reaction looks like, and I've tried two sources of Pyrrolidine. Must be my technique, or is this normal?

|
|
|
clearly_not_atara
International Hazard
    
Posts: 2787
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
I believe morpholine is used because other enamines are more likely to polymerize. The polymerized enamine may be what you are seeing.
|
|
|
Texium
|
Thread Moved
18-3-2017 at 21:59 |
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
you could try this method:
| Quote: | | A 50 mL round-bottomed flask containing cyclohexanone (3.82 g, 39 mmol) in anhydrous toluene (20 mL) was fitted with a Dean-Stark trap containing 3
Å molecular sieves, reflux condenser and a heating mantle, pyrrolidine (6.00 mL) was added, and the solution heated to reflux for 18 h. The solvent
was evaporated and the crude product 1-cyclohex-1-en-1-ylpyrrolidine was used directly for the next reaction. (6.0 g, 102% yield, 95% purity by HPLC).
MS(ESI+): 152.3; MS(ESI−): 150.1 |
https://www.google.com/patents/US8288432?cl=en10
[Edited on 19-3-2017 by CuReUS]
|
|
|
ovit.us
Harmless

Posts: 3
Registered: 18-3-2017
Member Is Offline
Mood: No Mood
|
|
What exactly is a Polymerized Enamine? Can I still use it?
My next step would be to wash with H2O to remove the catalyst acid, then separate, dry with Sodium Sulfate, and vacuum distill. This should produce a
colorless Enamine, but as mentioned, it later has turned yellow on me and not produced the desired reactions later on.
Quote: Originally posted by CuReUS  | you could try this method:
| Quote: | | A 50 mL round-bottomed flask containing cyclohexanone (3.82 g, 39 mmol) in anhydrous toluene (20 mL) was fitted with a Dean-Stark trap containing 3
Å molecular sieves, reflux condenser and a heating mantle, pyrrolidine (6.00 mL) was added, and the solution heated to reflux for 18 h. The solvent
was evaporated and the crude product 1-cyclohex-1-en-1-ylpyrrolidine was used directly for the next reaction. (6.0 g, 102% yield, 95% purity by HPLC).
MS(ESI+): 152.3; MS(ESI−): 150.1 |
https://www.google.com/patents/US8288432?cl=en10
[Edited on 19-3-2017 by CuReUS] |
The differences I see in this procedure are the absence of p-Toluenesulfonic-acid, addition of Molecular Sieves, and longer reaction time. I suppose
using the crude product is also something to consider.
|
|
|
zed
International Hazard
    
Posts: 2283
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Well, the reaction certainly works when Piperidine is employed as the amine.
Which has led to Piperidine becoming list one, and cyclohexanone becoming a special surveillance item.
[Edited on 28-3-2017 by zed]
[Edited on 28-3-2017 by zed]
|
|
|
Racconized
Harmless

Posts: 22
Registered: 27-11-2016
Member Is Offline
Mood: Yes
|
|
Looks like the procedure posted by xicori(may be wrong, can't remember his name exactly). Either way, maybe try going for the nitrile procedure
instead (if all else fails)
|
|
|