Loxia
Harmless

Posts: 1
Registered: 24-1-2016
Member Is Offline
Mood: No Mood
|
|
Interesting experiments with light?
Hi, I'd like to do some amazing experiments with light for my classmates and I'm looking for a good idea. It could be anything which have link between
chemistry and light (ideally if demonstration took about 15 minutes, for preparation I have 6-9 hours)
I already try some experiments, but more ideas will be very helpful. 
Thank you for inspiration.
[Edited on 18-3-2017 by Loxia]
|
|
|
j_sum1
Administrator
       
Posts: 6321
Registered: 4-10-2014
Location: At home
Member Is Offline
Mood: Most of the ducks are in a row
|
|
I think your field is wide open.
Lots of possibilities with photochemistry -- many reactions require UV radiation. (Not my field but you can look them up.) Early photography is full
of such things. I have worked with cyanotypes -- the original blueprints where exposure to daylight turns paper from yellow to blue in about four
minutes.
Also possible is thermochromism -- chemicals that change colour according to temperature. I came across duochromism the other day -- apparently
pumpkin seed oil changes colour according to how full the container is.
Then there are various chemiluminescent experiments you can do: chemical reactions that give off light. I understand that certain pool chemicals give
off red light with peroxide added. Nurdrage has a huge range of luminescent experiments on his YT channel -- including a recent posting of a
luminescent oscillating reaction. Most require luminol if you can get some.
Fluorescence is another possibility. Plenty of options. Shining a UV light on some peanut butter will cause it to glow temporarily.
Solvatochromism might appeal. Dissolve the juice from a highlighter pen in some methanol and in some water. Shine a UV light on it. The colour of
the fluorescing depends on the polarity of the solvent. (Works for fluorescein but not pyranine highlighters.) Pyranine fluoresces different colours
according to pH.
Of course you can't go past the good old flame test or if you are more sophisticated some spectroscopy. Light is given off in certain wavelengths
according to the excitation and de-excitation of electrons.
The bottom line is that chemistry s a pretty visual science. There is a lot going on between light and substances at an atomic level. Therefore
there are many ways you could approach this. You might want to be a bit more specific about your goals here -- are you interested only in emission of
light or is light-activation of a system appealing too? What constitutes success for your presentation?
That's just off the top of my head. Define a bit more narrowly what you are after and you have some pointers for beginning some research. Whatever
you choose you are not going to get away without doing a bit of reading. I know that there are many here who have dabbled with all kinds of light
experiments and have posted their findings. I am sure someone will help you with specific details or point you to a suitable thread once the range is
narrowed a bit.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
Aqueous suspensions of MgO and also ZnO are considered photo active and capable of creating reactive oxygen species (ROS) with even solar light.
ROS can be used to breakdown organic compounds, turn aqueous NH3 in the presence of air/O2 into small amounts of nitrate with time, partially
disinfect water with a combination of sunlight exposure, a touch of added NaCl, lemon juice, a copper salt and lots of vigorous air contact, to list
some possibilities.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
An unwanted CD or DVD can be used as a reflective diffraction grating; with a bit of bamboo or PVC pipe and some duct tape, you can easily make a
hand-held spectroscope. Pass it around; have your class use it to compare the continuous spectrum of an incandescent lamp to the discrete spectrum of
a fluorescent; explain the difference between emission and blackbody spectra. Or pass around a sample of spinach extract in a transparent vial; have
them compare the spectrum of raw sunlight to sunlight passed through the sample; the missing colors are the wavelengths absorbed by chlorophyll to
feed the plant.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Liamatpm
Harmless

Posts: 48
Registered: 7-12-2016
Location: Maine
Member Is Offline
Mood: Using the process of cellular respiration to stay (barely) alive
|
|
You could do an easy experiment on polarized light. (done with polarized and non polarized sunglasses)
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Edible diffraction gratings!
| Quote: |
The video starts with a basic discussion on the principles of diffraction gratings. The basis of the work is a commonly available diffraction grating,
readily available online. It’s a plastic sheet with thousands of microscopic ridges scored into the surface. The overarching method to create a
candy version of this is simple — coat the ridged surface in liquid chocolate or sugar syrup, to transfer the impression on to the candy surface
when it solidifies. However, the video goes further, explaining every step required to produce a successful end result. The attention to detail is on
the level of an industrial process, and shows a mastery of both science and candy processing techniques. If you’ve ever wondered how to properly
crystallize chocolate, this video has the knowledge you need. |
https://hackaday.com/2018/04/16/delicious-optics-a-chocolate...
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
LearnedAmateur
National Hazard
   
Posts: 513
Registered: 30-3-2017
Location: Somewhere in the UK
Member Is Offline
Mood: Free Radical
|
|
Diffraction gratings are always fun, we used to have these little square ‘windows’ in school with different values in lines per mm (we typically
used 80 I believe) for lasers so we could determine the wavelength of the light based on the angle/spacing between the maxima - basically just forms
an array of dots on the wall as the laser is split up. Oh yeah, and we also had this screw up thing where you create a tiny slit, maybe a 1-2mm or
less, and shining a laser through that does the same thing; we didn’t use that as per the curriculum but we had a play around with it anyway.
In chemistry, sometimes the solution is the problem.
It’s been a while, but I’m not dead! Updated 7/1/2020. Shout out to Aga, we got along well.
|
|
|
AJKOER
Radically Dubious
    
Posts: 3026
Registered: 7-5-2011
Member Is Offline
Mood: No Mood
|
|
An example of a longer term experiment would be using diffused sunlight versus direct light in the growth effect on plants.
See/research my recent comments/sources on 'diffused' light.
|
|
|
weilawei
Hazard to Others
  
Posts: 130
Registered: 3-12-2017
Member Is Offline
Mood: No Mood
|
|
Several years back, I spent a good amount of time on photolithography. You can make a cheap (somehwat toxic, be careful) resist using ammonium
dichromate and gelatin. Coat a copper plate with a thin layer, print a transparency sheet with the image you want to etch and place it over the plate,
then expose with UV light. Bake in an oven, wash off the unreacted resist with water, then etch in a bath of copper (II) chloride in hydrochloric acid
and a bit of hydrogen peroxide. Bubble air in to regenerate the bath.
You can use this for circuit boards, printing plates, etc..
Also, perhaps UV activated redox reactions in a test tube? Oh, and this looks neat: "Novel UV-Activated Colorimetric Oxygen Indicator".
| Quote: | | The results of a detailed characterization study of a novel UV-activated colorimetric oxygen indicator are described. The indicator uses nanoparticles
of titania to photosensitize the reduction of methylene blue by triethanolamine in a polymer encapsulation medium, using UVA light. Upon UV
irradiation, the indicator bleaches and remains in this colorless state in the dark, unless and until it is exposed to oxygen, whereupon its original
color is restored. The indicator is reusable and irreversible. The rate of color recovery is proportional to the level of oxygen present. A layer of
PET (poly(ethylene terephthalate)), of thickness b, placed on top of the indicator film slows down its response, and the 90% recovery time is
proportional to b. |
| Quote: | | Materials and Indicator Preparation. Unless stated otherwise, all chemicals were purchased from the Aldrich Chemical Co. (Gillingham, Dorset, UK). The
semiconductor, titanium dioxide (TiO2), was P25 provided by Degussa (Frankfurt, Germany). Degussa P25 TiO2 is a semiconductor that is commonly used in
photocatalyst research. It is a white powder comprising titania particles, typically ca. 30 nm in diameter, with an 80:20 anatase: rutile crystal
phase composition and an overall specific area of ca. 50 m2 g-1. All solutions were made up using doubly distilled, deionized water. All gases were
provided by BOC (London, U.K.). A typical example of the irreversible, reusable, UV-activated colorimetric oxygen indicator ink, used to make the
indicator films reported in this work, comprised: 5 g of a 5 wt % aqueous dispersion of P25 TiO2, 1 g of a 5 wt % aqueous solution of the redox
indicator dye, methylene blue (MB), 0.3 g of a mild sacrifical electron donor (SED), triethanolamine (TEOA), and 20 g of a 5 wt % aqueous solution of
an encapsulating polymer, hydroxyethyl cellulose (HEC). The indicator ink components were mixed together, subjected to 3 min ultrasound from an
ultrasound bath, to disperse the usually aggregated titania particles, and then stirred magnetically for 30 min to produce the final oxygen
intelligence ink for casting. A typical colorimetric oxygen indicator was prepared from this ink by placing 2-3 drops (ca. 0.1 mL) on to a 22 mm
diameter glass coverslip which was subsequently spun at 6000 rpm for 30 s. The final oxygen indicator film on the glass disk proved stable for over 1
year in the dark, under otherwise ambient conditions. The oxygen indicator films are blue and transparent, with absorbance maxima at 610 and 665 nm.
|
[Edited on 20-4-2018 by weilawei]
|
|
|
nezza
Hazard to Others
  
Posts: 324
Registered: 17-4-2011
Location: UK
Member Is Offline
Mood: phosphorescent
|
|
Fluorescence offers some interesting options. Europium salts fluoresce red under UV. Using a 405nm laser and Eu(III) solution the invisible beam
becomes visible.
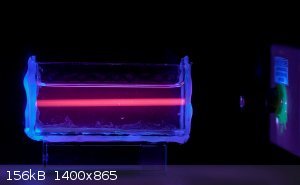
If you're not part of the solution, you're part of the precipitate.
|
|
|