Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Citric acid to Isopropanol. Possible method?
Hello,
Few weeks ago, I noticed that when you remove all CO2 from citric acid, you'll get isopropanol.
Would the classical way of decarboxylation work?
C6H8O7 + 3 NaOH ----> C6H5O7Na3 + 3 H2O
C6H5O7Na3 + 3 NaOH ----> 3 Na2CO3 + (CH3)2COH
I didn't find any info about this on google, but if my procedure works, then it is an easy way to homemade IPA.
Rest In Pieces!
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Sounds interesting but what conditions do you need to decarboxylate with NaOH? Because if you need to heat it, wikipedia says that the decomp temp =
175 °C
which is very low.
Also, there are 3 COOH groups that need to be removed. That would probably effect the yield.
|
|
|
Adas
National Hazard
   
Posts: 711
Registered: 21-9-2011
Location: Slovakia
Member Is Offline
Mood: Sensitive to shock and friction
|
|
Note that in the reaction, that needs heating, uses sodium citrate which melts at 300°C 
Rest In Pieces!
|
|
|
GreenD
National Hazard
   
Posts: 623
Registered: 30-3-2011
Member Is Offline
Mood: Not really high anymore
|
|
From my experience working with these small organic poly-acids, is you're going to get a very sticky brown/orange mess.
We used to decarboxylate citric acid (when I wasn't busy eating it) in benzene (we also tried xylene and toluene) at high temps. We were trying to get
a route to lactic acid to make PLA. The decarboxylation worked well - eventually everything would go into solution after about 24 hours of reflux, a
nice yellow solution.
We attempted to extract, but for some reason slight basification yielded some messy product (can't remember exactly what happened).
Distillation provided char, often.
Most importantly - you will not get IPA. The hydroxy group gets knocked off and water forms. We used a modified dean-stark trap to remove water, to
prevent the reversal, so I'm not sure what the equilibrium constant is (whether you'll get more hydroxy or more water). Our main product was maleic
acid. however - as above, vacuum distillation produced tar, and base extraction yielded a mess (but I can't remember why!)
In short: this is not a feasible route to IPA. IPA is cheap, buy it at the store.
[Edited on 17-2-2012 by GreenD]
|
|
|
Nicodem
|
Thread Moved
18-2-2012 at 11:27 |
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Frankly, if you want IPA, as GreenD says just purchase it online or as either a solvent or rubbing alcohol. Citric acid is probably much more
valuable.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Geocachmaster
Hazard to Others
  
Posts: 146
Registered: 5-3-2016
Location: Maine, USA
Member Is Offline
Mood: Corroded, just like my spatulas
|
|
Despite GreenD saying this would not work, I decided to try it anyway. After all, aga is always complaining about too much talk and not enough
chemistry . IPA is super cheap, much cheaper that citric acid, so this was only a
proof of concept and you should just buy some at the store if it's needed. . IPA is super cheap, much cheaper that citric acid, so this was only a
proof of concept and you should just buy some at the store if it's needed.
Intended reaction: one mole citric acid + heat → one mole IPA + three moles CO2
20.01g of anhydrous citric acid was added to a 100ml round bottom flask. This represents a .104 molar scale. The flask was fitted with a short path
condenser (for ease of use, not efficiency) and placed in a salt bath. A thermometer was added to the salt bath and heating commenced. 5 minutes of
heating brought the bath to ~165C when the contents of the flask began to liquefy. The reaction mixture was colorless and slowly bubbling at this
point. Condensation was observed in the flask and still head. 4 minutes later it had completely liquified and was bubbling more vigorously, getting
first drops in the receiving flask. 10 minutes passed collecting distillate while the contents of the flask had slowly become a dark yellow/orange.
Soon after small amounts of yellowish liquid started to come over and white wispy vapors were seen in the still head, heating was stopped. Dark,
orange, viscous liquid was left in the distillation flask and very slightly yellow liquid was in the receiving flask.
The product was approximately 4 ml of liquid having a mass of 3.84g. It had a distinct odor of IPA and rubbing alcohol. It was found to be flammable
and had a flammability close to that of 65% w/w isopropyl alcohol in water (found through experimentation).
3.84g of 65% IPA is 2.5g of the pure alcohol. Assuming what came over was only Isopropanol and water, 2.5g / 6.26g theoretical yield is a 39.9% yield.
This procedure could definitely be optimized and changed to get higher yields, but it would not be worth it, other than for the fun of it. For example
the short path condenser did not condense all of the vapors coming over. The method of finding the yield was very likely inaccurate but that was
deemed unimportant because the experiment was only meant to find out if any isopropanol was made.
 
The setup and partway through the reaction.
The salt bath works pretty good!
 
At the end

The product burning, sorry my camera is awful in the dark...
|
|
|
Amos
International Hazard
    
Posts: 1406
Registered: 25-3-2014
Location: Yes
Member Is Offline
Mood: No
|
|
Add some of the distillate to water and use pH paper or some indicator to see if the pH is changed substantially. You may have some organic acids as
byproducts.
If you've got largely IPA in there, it should separate out from any water if you saturate with sodium hydroxide.
If you want to confirm once and for all that you have a secondary alcohol there, you may want to try testing with Lucas' reagent, but this may require
more of your distillate than you currently have.
[Edited on 2-9-2017 by Amos]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
So as a pedagogical exercise, in theory one could probably produce ethanol from malic acid as well, and also perhaps n-propyl amine from glutamic
acid.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I don't understand what you mean by all this talk about 2-propanol. There is no reference, or anything else in this thread, that would suggest
2-propanol as a possible product from citric acid thermolysis.
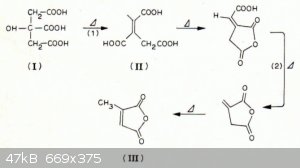
Thermal decomposition of citric acid is well known to give a mixture of products (the so called "pyrocitric acids"), composed mainly of citraconic
acid, itaconic acid, mesaconic acid, 3-hydroxyglutaric acid, their cyclic anhydrides and other stuff. On the other hand, you offer no indication that
the distillate contains 2-propanol (flammability is no specific property of this alcohol). And anyway, what would the mechanism of its formation be?
A scheme from DOI: 10.1016/0040-6031(86)87081-2
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
JJay
International Hazard
    
Posts: 3440
Registered: 15-10-2015
Member Is Offline
|
|
A quick way to check to see if it contains a significant amount of isopropanol would be to redistill it and check the boiling point. Of course, if any
of the products have similar boiling points to isopropanol, the results might be confounding.
Neutralizing any acids present would help to eliminate potential confounders. I'm pretty sure that strong sodium hydroxide solution would break up
those anhydrides (use eye protection, gloves, respirator if dealing with its powdered form, lab coat, etc.).
[Edited on 9-2-2017 by JJay]
|
|
|
PirateDocBrown
National Hazard
   
Posts: 570
Registered: 27-11-2016
Location: Minnesota
Member Is Offline
Mood: No Mood
|
|
Well, I don't know about this exactly. But it would occur to me that there are 2 decarboxylations happening here, the ones for the terminal acids, and
the one in the middle of the chain. Different conditions might be optimal for each.
That said, I wrote my first paper in grad school on the body's use of iron as a catalyst for the metabolism of citrate.
Some experimentation using iron, or some other transition metal, might not go awry here.
|
|
|
Fluorite
Hazard to Others
  
Posts: 139
Registered: 26-12-2018
Location: United Arab Emirates
Member Is Offline
|
|
OMG Geocachmaster is a life saver! I wanted IPA to make luminol but I didn't find any! It always mixed with ethyl acetate and acetone in a 6$ 70ml
nail Polish remover :/
Citric acid can be veryy cheap if you extract it from rotten lemons OR just heat lemon juice to dryness and pyrolyse the product directly?
|
|
|
Bedlasky
International Hazard
    
Posts: 1242
Registered: 15-4-2019
Location: Period 5, group 6
Member Is Offline
Mood: Volatile
|
|
Decomposition with ferric ions under UV light should work. Each mole of citric acid release three moles of CO2 in this reaction.
|
|
|
macckone
Dispenser of practical lab wisdom
    
Posts: 2168
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
decomposition reactions are always messy.
Pyrolysis of wood as a classic example.
The primary products are carbon and water because wood is primarily carbohydrates and lignin.
But the by products are acetic acid, formic acid and methanol as well as many other compounds.
The methanol produced is actually rather low but it is usable.
IPA may not be the main product but it could still be a significant one.
For a while isopropyl alcohol could not be had for any price outside of a hospital and we are headed there again so this may be useful.
Of course this is science and it must be replicated.
This study decomposed citric acid but was looking at the carbon products:
https://link.springer.com/article/10.1007/s40097-017-0222-9
and another:
https://www.nature.com/articles/s41598-017-11572-8#:~:text=D...
If the by-product is the valuable quantum carbon dots, then yes this may be practical and increase the financial returns.
So yes it may be feasible to do this and even financially so.
|
|
|